Catheter innovations have had a remarkable year so far, with many receiving regulatory approval as they prepare to enter the market. The pulsed field ablation market heated up in 2023 and continues to make waves this year with more companies entering the space. Intravascular lithotripsy is having its moment as well – Shockwave Medical was … [Read more...] about The top catheter-based innovation news stories of 2024 — so far
Biosense Webster
Biosense Webster reports positive dual-energy ablation results
Johnson & Johnson's Biosense Webster unit today announced positive three-month follow-up results from its SmartfIRE clinical trial. The study evaluated the use of the dual-energy ThermoCool SmartTouch SF catheter. ThermoCool SmartTouch SF is the first dual-energy pulsed field ablation (PFA)/radiofrequency (RF) ablation catheter integrated … [Read more...] about Biosense Webster reports positive dual-energy ablation results
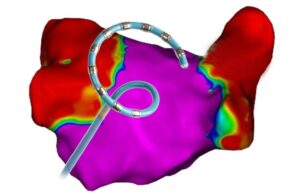
High voltage in the heart: PFA catheter design tips from Biosense Webster
Tushar Sharma at J&J Medtech’s Biosense Webster discusses pulsed-field ablation (PFA) catheter shapes, sensors, electrodes and device design tips.
Biosense Webster’s pulsed-field ablation (PFA) catheter design and development starts in the heart.
That’s where PFA treats atrial fibrillation (AFib) by killing cardiac cells to isolate errant heart signals and prevent them from triggering irregular heartbeats. PFA uses electric fields to permanently open holes in the walls of heart cells … [Read more...] about High voltage in the heart: PFA catheter design tips from Biosense Webster
Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
Johnson & Johnson MedTech's Biosense Webster today announced Japanese approval for its Varipulse platform for treating AFib. Varipulse treats symptomatic drug-refractory recurrent paroxysmal AFib using pulsed field ablation (PFA). The platform features the Varipulse catheter, a variable-loop multielectrode catheter, the TruPulse generator … [Read more...] about Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Johnson & Johnson's Biosense Webster today announced the first completed patient cases in a study of its dual-energy ablation catheter. Biosense Webster designed the ThermoCool SmartTouch SF to deliver both radiofrequency (RF) and pulsed-field ablation (PFA) energy. The SmartPulse pivotal study evaluates the dual-energy system in the … [Read more...] about J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Biosense Webster has positive data for QDOT Micro ablation catheter
Johnson & Johnson MedTech's Biosense Webster today announced new findings supporting the use of its QDOT Micro catheter. Published in the Journal of Cardiovascular Electrophysiology, the results demonstrate improved AFib control. They also showed relief of symptoms and overall quality of life improvements with QDOT Micro ablation … [Read more...] about Biosense Webster has positive data for QDOT Micro ablation catheter
Biosense Webster: catheter ablation leads to lower heart failure risk in AFib patients
Johnson & Johnson's Biosense Webster today announced data supporting the use of catheter ablation in AFib patients. The study, funded by the Irvine, California-based company, looked at heart failure incidence risks in AFib patients. It compared the use of catheter ablation versus antiarrhythmic drugs (AAD). Looking at non-specific catheter … [Read more...] about Biosense Webster: catheter ablation leads to lower heart failure risk in AFib patients
Biosense Webster begins Omnypulse pulsed-field ablation trial
Johnson & Johnson's Biosense Webster today announced the first cases with its investigational Omnypulse catheter as part of the Omny-IRE clinical trial. The Omnypulse platform features the Omnypulse catheter and the Trupulse generator. Omny-IRE looks at the platform for mapping and treatment of symptomatic paroxysmal AFib during standard … [Read more...] about Biosense Webster begins Omnypulse pulsed-field ablation trial
J&J used RWE for expanded indications — and you can, too
Two J&J MedTech leaders shared advice to help medical device developers use real-world evidence (RWE) in FDA submissions. Real-world evidence (RWE) took a big step forward recently when the FDA approved expanded indications for Johnson & Johnson MedTech ablation catheters. For the first time, the federal medical device safety regulator … [Read more...] about J&J used RWE for expanded indications — and you can, too