Two J&J MedTech leaders shared advice to help medical device developers use real-world evidence (RWE) in FDA submissions. Real-world evidence (RWE) took a big step forward recently when the FDA approved expanded indications for Johnson & Johnson MedTech ablation catheters. For the first time, the federal medical device safety regulator … [Read more...] about J&J used RWE for expanded indications — and you can, too
ablation
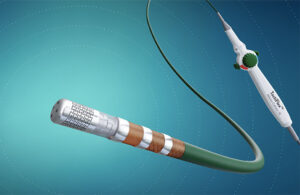
Abbott wins FDA approval for next-gen TactiFlex ablation catheter
Abbott (NYSE: ABT) today announced FDA approval of its TactiFlex Ablation Catheter, Sensor Enabled, which the company describes as the world's first ablation catheter with a flexible tip and contact force technology. The TactiFlex catheter also integrates with Abbott's EnSite X EP System, enabling physicians to visualize heart anatomy more … [Read more...] about Abbott wins FDA approval for next-gen TactiFlex ablation catheter