This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
Implants
The biggest cardiovascular stories from TCT 2023
Every year, some of the biggest names in cardiovascular technologies come together in one place for TCT. This year's 35th edition of the Transcatheter Cardiovascular Therapeutics annual scientific symposium was no different in San Francisco. Usual suspects like Medtronic and Abbott had data on display for a range of products, while other big … [Read more...] about The biggest cardiovascular stories from TCT 2023
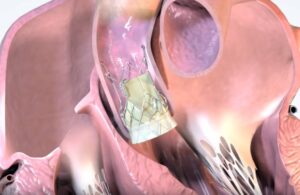
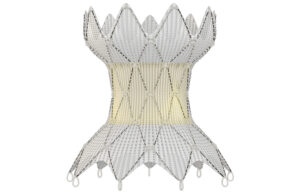
Onecrea Medical completes first-in-human implants of TAVR system
Onecrea Medical recently announced that it successfully completed first-in-human implants for its transcatheter aortic valve replacement (TAVR) system. The company designed its next-generation TAVR system to treat aortic stenosis. With a unique concave and convex structure, the TAVR system anchors the valve effectively in the aortic annulus. … [Read more...] about Onecrea Medical completes first-in-human implants of TAVR system
After recall and relaunch, Medtronic wants to go global with its catheter-delivered Harmony valve
Each Medtronic Harmony valve is sewn by hand to attach laser-cut pig tissue to the nitinol that makes this minimally invasive heart implant possible. Medtronic’s Harmony transcatheter pulmonary valve (TPV) design is paying off after engineers solved a delivery catheter recall and relaunched the system this year. The Harmony TPV uses pig tissue, … [Read more...] about After recall and relaunch, Medtronic wants to go global with its catheter-delivered Harmony valve
How Medtronic uses nitinol to improve the structure and effectiveness of heart devices
Tim Laske, VP of research and business development for Medtronic‘s cardiac ablation solutions business, discusses the challenges of designing devices for the heart and explores the properties of nitinol. The heart is often one of the most underappreciated aspects of human anatomy, and its atrial appendages are often overlooked even in cardiac … [Read more...] about How Medtronic uses nitinol to improve the structure and effectiveness of heart devices
10-year data shows durable outcomes with Medtronic Endurant stent graft system
Medtronic (NYSE:MDT) announced positive results from its 10-year post-market registry for the Endurant stent graft. The Endurant stent graft system offers Endovascular aneurysm repair (EVAR) to treat patients with abdominal aortic aneurysm (AAA). Medtronic posted the real-world data at the 2023 Charing Cross Symposium in London, marking the … [Read more...] about 10-year data shows durable outcomes with Medtronic Endurant stent graft system
The top 10 catheter innovation news stories of 2022
This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022
The 24 best medical device innovations of 2022
The Galien Foundation recently announced its nominees of medical device innovations for its 2022 Prix Galien USA awards. There are 24 medical technologies nominated for the annual award this year, up from 18 nominees in 2021. The Galien Foundation’s annual Prix Galien awards highlight devices, biotechnology and pharmaceutical products … [Read more...] about The 24 best medical device innovations of 2022
Cardio Flow’s FreedomFlow guidewire wins FDA clearance
Cardio Flow this week won FDA 510(k) clearance for its FreedomFlow peripheral guidewire and announced the first commercial case using the device in the U.S. St. Paul, Minnesota–based Cardio Flow designed FreedomFlow to provide exceptional support for diagnostic and therapeutic devices in treating plaque blockages in arteries above and below the … [Read more...] about Cardio Flow’s FreedomFlow guidewire wins FDA clearance
18 of the world’s most innovative medical technologies
Judges this week are voting for the most innovative medical technologies among the nominees for the 2022 Prix Galien International Awards. The Prix Galien International Awards highlight the most innovative technologies in the life sciences industry. The New York-based Galien Foundation presents the international award to eligible nominees that … [Read more...] about 18 of the world’s most innovative medical technologies