Alcon (NYSE:ALC) announced today that it completed its $475 million acquisition of glaucoma surgery device maker Ivantis. Geneva, Switzerland-based Alcon initially announced the acquisition of Ivantis, which develops the Hydrus Microstent minimally invasive glaucoma surgery (MIGS) device, in November 2021. According to a news release, the … [Read more...] about Alcon closes Ivantis acquisition
Implants
Medtronic Evolut Pro TAVR wins approval in China
Medtronic today announced that it received National Medical Products Administration approval in China for its CoreValve Evolut Pro TAVR system. The approval marks the first Medtronic TAVR system approved in China for patients with symptomatic severe aortic stenosis who are at high or extreme risk for open-heart surgery, according to the … [Read more...] about Medtronic Evolut Pro TAVR wins approval in China
Foldax gets greenlight in India for biopolymer surgical aortic heart valve clinical trial
Foldax this week announced that India's medical device regulatory body approved initiation of a clinical trial to study its Tria biopolymer surgical aortic heart valve. Salt Lake City-based Foldax designed the Tria heart valve to resist calcification, withstand stresses and strains without failure and restore patient quality of life without the … [Read more...] about Foldax gets greenlight in India for biopolymer surgical aortic heart valve clinical trial
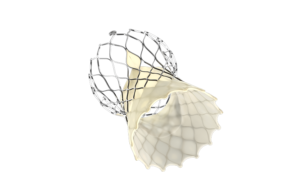
Edwards touts trial results for Evoque transcatheter tricuspid valve replacement system
Edwards Lifesciences (NYSE:EW) shared data from a clinical trial showing that its Evoque transcatheter tricuspid valve replacement offers favorable patient outcomes. Irvine, California–based Edwards presented the data during the late-breaking clinical science session at the 33rd Transcatheter Cardiovascular Therapeutics (TCT), according to a … [Read more...] about Edwards touts trial results for Evoque transcatheter tricuspid valve replacement system
3 pitfalls to consider when creating catheter delivery systems
Multiple components go into a catheter delivery system, with several ways to combine them. An Edwards Lifesciences senior R&D director goes over potential pitfalls. A catheter is a tube that can deliver devices or pharmaceuticals into the body. It is also commonly used for diagnostic purposes. A catheter has a hub or a handle on its end … [Read more...] about 3 pitfalls to consider when creating catheter delivery systems
FDA approves Medtronic next-gen Evolut FX TAVR system
Medtronic today announced that its Evolut FX TAVR system has won FDA approval. Fridley, Minn.–based Medtronic designed the self-expanding transcatheter aortic valve replacement system to treat symptomatic severe aortic stenosis. The TAVR system uses a supra-annular valve design that has demonstrated hemodynamic performance that is superior to … [Read more...] about FDA approves Medtronic next-gen Evolut FX TAVR system
FDA grants breakthrough device designation for Abiomed’s Impella ECP heart pump
Abiomed (NSDQ:ABMD) this week announced that the FDA has granted breakthrough device designation for its Impella ECP expandable percutaneous heart pump. Danvers, Mass.-based Abiomed designed the Impella ECP heart pump to be compatible with small-bore access and closure techniques. It measures 3 mm in diameter when inserted and removed from the … [Read more...] about FDA grants breakthrough device designation for Abiomed’s Impella ECP heart pump
FDA approves Abbott Amplatzer Amulet to treat AFib
Abbott this week announced that it has received FDA approval for its Amplatzer Amulet device to treat atrial fibrillation. The Amplatzer Amulet is a left atrial appendage occluder for treating atrial fibrillation (AFib) with a risk of ischemic stroke. The device is a small pouch connected to the upper left chamber of the heart. Get the full … [Read more...] about FDA approves Abbott Amplatzer Amulet to treat AFib
The 18 most innovative medical devices of 2021
The Galien Foundation today announced the nominees for most innovative medical devices for its 15th annual Prix Galien USA Awards. The foundation nominates devices, biotechnology and pharmaceutical products for its annual Prix Galien awards to highlight products designed to improve the human condition. “The Awards Committee is excited to … [Read more...] about The 18 most innovative medical devices of 2021
Glaukos wins Australian regulatory approval for MicroShunt
Glaukos (NYSE:GKOS) recently announced that it received regulatory approval from Australia's Therapeutic Goods Administration for its Preserflo MicroShunt. The San Clemente, Calif.–based company designed the MicroShunt to reduce intraocular pressure in the eyes of patients who have primary open-angle glaucoma where IOP remains uncontrollable or … [Read more...] about Glaukos wins Australian regulatory approval for MicroShunt