Each Medtronic Harmony valve is sewn by hand to attach laser-cut pig tissue to the nitinol that makes this minimally invasive heart implant possible.
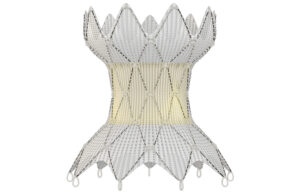
The Harmony TPV uses pig tissue, superelastic nitinol and manufacturing techniques old and new to solve a special challenge for children and adults.
The catheter-placed Harmony valve offers a minimally invasive way to improve the flow of blood to the lungs and delay open-heart surgery for congenital heart disease. Congenital heart defects are present in about 40,000 babies born each year, making it the most common type of birth defect.
Harmony TPV is for patients with right ventricular outflow tract (RVOT) anomalies and severe pulmonary valve regurgitation. After the heart pumps deoxygenated blood into the lungs, that blood leaks back into the heart’s right lower chamber instead of being pumped out to the rest of the body.
“It can start as a small leak, but the blood starts going backward through the valve … and starts backing up within the body,” Garrett Pilcher, Medtronic VP of clinical research for Structural Heart, said in an interview with Medical Design & Outsourcing.
Patients will start feeling sluggish and face a variety of infections and other problems due to lack of oxygenated blood flow through the body.
“Harmony was the first device approved by FDA for this indication, and before this patients were getting multiple procedures over time, ultimately open-heart surgery,” he said. “… It was a first-of-a-kind design to get into the right ventricular outflow tract for these patients that didn’t have a minimally invasive option prior to that.”
Medtronic recently released two-year results for 86 patients with Harmony TPV implants. About half received the 22 mm valves and the other half received 25 mm valves. The study showed no vascular injuries requiring intervention and only 3% of patients with more than mild pulmonary regurgitation.
“We’re not seeing severe backflow, which was the original issue we were trying to solve,” Pilcher said.
Though surgeons will want to see 10 or 15 years of durability to compare Harmony against open-heart surgery, the study shows at least two years of durability — and counting.
“We’re seeing no detriment or the valve starting to not function as well,” Pilcher said.

