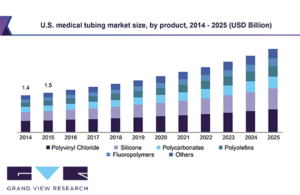
The medical tubing market is expected to be worth $11.9 billion by 2025, according to an analysis from Grand View Research. Grand View Research suggests the industry will expand at a revenue-based consumer annual growth rate of 9.2% over the next five years. Increased health expenditures from individuals and increased emphasis on healthcare by … [Read more...] about Medical tubing market to be worth $12 billion in 2025
Implants
LivaNova pulls the plug on transcatheter mitral valve repair device
LivaNova (NSDQ:LIVN) said recently that it is ending its Caisson Interventional transcatheter mitral valve replacement (TMVR) program and plans to restructure its heart valve business to improve profitability. The company’s heart valve business represented nearly $130 million in revenue during fiscal 2018 and saw its revenue numbers decline over … [Read more...] about LivaNova pulls the plug on transcatheter mitral valve repair device
Study: Boston Sci’s Watchman cheaper than drugs long-term
A new study has found that use of the Boston Scientific (NYSE:BSX) Watchman left atrial appendage closure (LAAC) device to treat atrial fibrillation costs less than existing and novel anticoagulant drugs over five years. The retrospective study pooled data from the randomized trials of the Marlborough, Mass.-based company’s Watchman device. The … [Read more...] about Study: Boston Sci’s Watchman cheaper than drugs long-term
CMS expands TAVR coverage
The Centers for Medicare and Medicaid Services have updated the criteria for hospitals and physicians to begin or continue providing transcatheter aortic valve replacement surgery. The decision gives hospitals and providers more flexibility to meet the CMS requirements for performing TAVR, the agency said Friday. The original National Coverage … [Read more...] about CMS expands TAVR coverage
Beaumont Machine relocates, starts new chapter
Beaumont Machine has opened a new manufacturing plant in Batavia, Ohio under the renewed leadership of its founder. Beaumont's electrical discharge machining (EDM) machines are used primarily for precision placement of the cooling and gas flow holes in various products, including metering and diffuser holes. They are used extensively in the … [Read more...] about Beaumont Machine relocates, starts new chapter