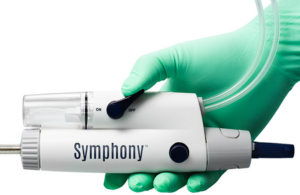
Imperative Care announced today that it completed enrollment in the SYMPHONY-PE pivotal investigational device exemption (IDE) study. The FDA IDE study evaluates the company's Symphony Thrombectomy System for treating acute pulmonary embolism (PE). Designed to expand Symphony's current indication for use to include PE, investigators conducted … [Read more...] about Imperative Care completes enrollment in pulmonary embolism study
Imperative Care
Imperative Care expands Zoom Stroke System with new dual-catheter launch
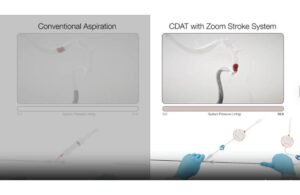
Imperative Care announced today that it expanded its Zoom Stroke System with the launch of its Zoom DuoPort technology. Zoom DuoPort allows physicians to continue using the novel continuous dual-aspiration technique (CDAT) on two Zoom aspiration catheters simultaneously from a single vacuum source. CDAT enables enhanced aspiration control and … [Read more...] about Imperative Care expands Zoom Stroke System with new dual-catheter launch
Imperative Care to kick off new trial of Zoom stroke treatment
Imperative Care announced today that it intends to fund a trial comparing its Zoom stroke system plus best medical treatment to treatment alone. Campbell, California–based Imperative Care designed Zoom for fast and effective clot removal from access through reperfusion. It treats patients with acute ischemic stroke, enabling smooth tracking … [Read more...] about Imperative Care to kick off new trial of Zoom stroke treatment
FDA clears Imperative Care’s Symphony 16F 82cm catheter
Imperative Care today announced FDA 510(k) clearance of its Symphony 16 Fr 82cm catheter for venous thrombosis and announced the first successful cases. Campbell, California-based Imperative Care said the catheter joins the Symphony Thrombectomy System's 16 Fr 117 cm and 24 Fr 85 cm catheters. "The customized length of the 16 Fr 82 cm … [Read more...] about FDA clears Imperative Care’s Symphony 16F 82cm catheter
Imperative Care wins FDA clearance for Zoom stroke treatment
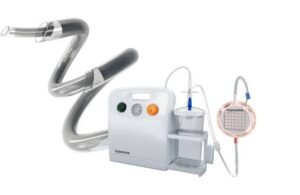
Imperative Care announced today that it has secured FDA 510(k) clearance for its Zoom comprehensive stroke thrombectomy system. According to Campbell, California–based Imperative Care, the Zoom system is the first such system to include large-bore .088-in. catheters indicated for both access and aspiration when used with one of the company's … [Read more...] about Imperative Care wins FDA clearance for Zoom stroke treatment
Imperative Care has positive stroke treatment data
Imperative Care today announced positive data from a study of its Zoom reperfusion system for the treatment of ischemic stroke. Dr. William Mack, co-principal investigator, presented the results at the Society of NeuroInterventional Surgery (SNIS) annual meeting. The study evaluated 260 patients across 26 U.S. institutions between … [Read more...] about Imperative Care has positive stroke treatment data
Imperative Care wins FDA nod for stroke catheter
Imperative Care announced today that the FDA granted 510(k) clearance for its Zoom 6F insert catheters for ischemic stroke procedures. The company also announced the successful completion of the first case with the Zoom 6F insert catheters. Dr. Dana Tomalty performed the case at Huntsville Hospital in Huntsville, Alabama. Tomalty used the … [Read more...] about Imperative Care wins FDA nod for stroke catheter
The top 10 catheter innovation news stories of 2022
This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022