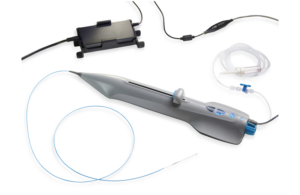
St. Paul, Minnesota–based Cardio Flow designed FreedomFlow to provide exceptional support for diagnostic and therapeutic devices in treating plaque blockages in arteries above and below the knee. It has a stainless steel core-to-tip design with a fixed distal-spring coil, both of which are silicone-coated.
The silicone coating eases the crossing of difficult blockages. Its 0.014 in. core-to-tip design gives interventionists torque transmission and precise control when delivering therapeutic devices.
Dr. Jihad Mustapha performed the first procedure using the device at Advanced Cardiac and Vascular Centers for Amputation Prevention in Grand Rapid, Michigan. FreedomFlow was used on a patient presenting with complex multi-vessel disease with blockages below the knee, requiring a pedal loop.
“I was able to deliver three PTA balloons, one IVUS catheter, and a therapeutic device across the single guidewire—all glided easily along the FreedomFlow wire. This new product has the potential to save time and costs in the endovascular lab, given its versatility in crossing occlusions and ability to deliver multiple interventional devices on a single guidewire,” Dr. Mustapha said in a news release.

