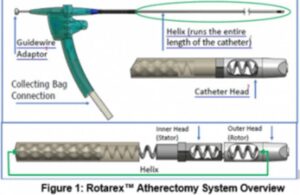
BD [WtwhTicker symbol="BDX"](NYSE: BDX)[/WtwhTicker] announced today that it plans to initiate a patient data registry for its Rotarex atherectomy system. The registry aims to measure real-world outcomes for patients with peripheral artery disease (PAD). XTRACT, a multi-center, single-arm, post-market registry assesses Rotarex's clinical … [Read more...] about BD to begin real-world atherectomy registry for PAD
BD
BD warns on esophagogastric balloon tamponade tubes after death
The FDA issued a notice highlighting a letter issued by BD and its C.R. Bard Urology and Critical Care subsidiary warning of new balloon device instructions. This warning follows one death associated with the device issue. BD sent affected customers a notice updating instructions for all lots of certain esophagogastric balloon tamponade tubes. … [Read more...] about BD warns on esophagogastric balloon tamponade tubes after death
BD warns on intravascular catheter
The FDA shared an alert that BD (NYSE: BDX) and its Bard subsidiary warned customers of an issue with certain intravascular catheters. BD recommended the removal of certain unused PowerPICC intravascular catheters from use or sale. In-use PowerPICC intravascular catheters also received updated instructions for use. Affected products include the … [Read more...] about BD warns on intravascular catheter
BD warns on atherectomy catheters after deaths
BD (NYSE: BDX) recently issued a medical device correction later related to its Bard subsidiary's Rotarex atherectomy system. The company identified a number of anatomical factors that could contribute to catheter helix fracture and/or breakage. It reports 30 serious injuries and four deaths associated with the issue. Additionally, BD reported … [Read more...] about BD warns on atherectomy catheters after deaths
BD increases U.S. syringe, IV catheter manufacturing
BD (NYSE:BDX) announced today that it plans to invest further in U.S. manufacturing to add capacity for certain critical medical devices. Those medical devices include syringes, needles and IV catheters. The Franklin Lakes, New Jersey-based company aims to meet the ongoing needs of the nation's healthcare system. This uptick in manufacturing … [Read more...] about BD increases U.S. syringe, IV catheter manufacturing
Zeus hires new CEO from Becton Dickinson
Zeus has hired Padraic “Paddy” O’Brien as CEO from Becton, Dickinson and Co. effective April 1, the medical tubing supplier said today. O’Brien is worldwide president of BD Peripheral Intervention, where he “developed an extensive and consistent track record of delivering durable, sustainable growth while creating a winning culture,” Zeus said … [Read more...] about Zeus hires new CEO from Becton Dickinson
BD initiates IDE trial of stent for treating peripheral arterial disease
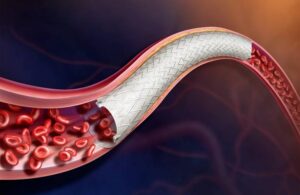
BD (NYSE: BDX) announced today that it enrolled the first patient in a trial of its Vascular Covered Stent for treating peripheral arterial disease (PAD). Franklin Lakes, New Jersey–based BD is evaluating the safety and effectiveness of the stent in its AGILITY FDA investigational device exemption (IDE) trial. The self-expanding, low-profile, … [Read more...] about BD initiates IDE trial of stent for treating peripheral arterial disease
BD launches SiteRite vascular access ultrasound system
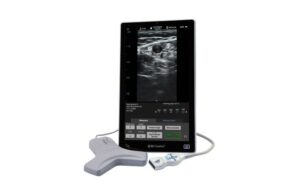
BD (NYSE:BD) announced today that it launched its new SiteRite 9 advanced ultrasound system for helping with vascular access device placement. Franklin Lakes, New Jersey-based BD designed the system to help improve clinician efficiency when placing these devices. They include peripherally inserted central catheters (PICCs), central venous … [Read more...] about BD launches SiteRite vascular access ultrasound system
BD enrolls first patient in study of stent graft for treating portal hypertension
BD (NYSE:BDX) announced today that it completed the enrollment of the first patient in a trial of its Liverty TIPS stent graft. Franklin Lakes, New Jersey-based BD's ARCH FDA investigational device exemption (IDE) study evaluates the safety and effectiveness of Liverty. The global, prospective, multi-center, single-arm clinical study looks at … [Read more...] about BD enrolls first patient in study of stent graft for treating portal hypertension
BD expands availability of all-in-one flush pre-filled syringe
BD (NYSE:BDX) announced today that it expanded customer availability for its all-in-one pre-filled flush syringe. Franklin Lakes, New Jersey-based BD designed its PosiFlush SafeScrub syringe with an integrated disinfection unit. It reinforces compliance to infection prevention guidelines, simplifying nursing workflow. BD built the active … [Read more...] about BD expands availability of all-in-one flush pre-filled syringe