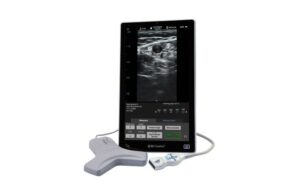
Franklin Lakes, New Jersey-based BD designed the system to help improve clinician efficiency when placing these devices. They include peripherally inserted central catheters (PICCs), central venous catheters, IV lines and other vascular access devices. SiteRite 9 received FDA 510(k) clearance in September.
The company designed SiteRite 9 as an all-in-one system with a user-friendly experience delivered through an updated 15.6-inch touchscreen. The screen features enhanced image quality with leading catheter placement tools and technologies. BD’s system includes integrated visualization tools like the Cue needle tracking system and Sherlock 3CG+ tip confirmation system. These provide continuous, real-time needle tracking and proper catheter tip navigation and location, respectively.
SiteRite 9 also features smart, connected technology, BD says. This includes vessel assessment tools that automatically detect the vessel and pair with measurement tools. This helps clinicians select the appropriate catheter and make an informed decision every time.
Additional capabilities include patient data lookup with information capture, auto-filling capabilities and record transfers. The system comes as part of BD’s “One-Stick Hospital Stay” initiative. BD announced this initiative more than two years ago in an effort to transform the patient experience.
“Our latest innovation in ultrasound technology is designed to make vascular access care delivery easier and more efficient for clinicians — whether they’ve been on the job for years or are new to the field,” said Eric Borin, BD worldwide president of Medication Delivery Solutions. “By combining our decades of leadership in vascular access with voice-of-the-customer enhancements, we’ve developed a best-in-class solution to aid in insertion success on the first attempt and reduce the need for repeated needlesticks to deliver a better care experience for everyone involved.”

