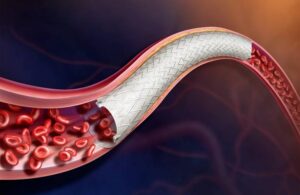
BD (NYSE: BDX) announced today that it enrolled the first patient in a trial of its Vascular Covered Stent for treating peripheral arterial disease (PAD).
Franklin Lakes, New Jersey–based BD is evaluating the safety and effectiveness of the stent in its AGILITY FDA investigational device exemption (IDE) trial. The self-expanding, low-profile, polytetrafluoroethylene encapsulated nitinol implant is deployed through a delivery system. This system provides controlled stent release, according to a news release.
BD plans for the trial to include 315 patients across up to 40 clinical study sites in the U.S., Europe, Australia and New Zealand. It expects follow-up for all treated patients at various points, starting at one month and ending at 36 months.
Dr. Nicolas Shammas enrolled the first patient in the study at Trinity Medical Center in Bettendorf, Iowa. Dr. Sean Lyden of the Cleveland Clinic serves as principal investigator for the study. Lyden highlighted the importance of the BD stent’s durability as it tracks to the lesion and opposes the vessel wall, in particular.
“There continues to be significant unmet needs in the treatment of PAD patients,” said Tim Hug, VP and GM at BD Peripheral Intervention. “We are excited to have initiated this study and evaluate the treatment benefits of this potentially differentiated technology. This stent could give interventionalists an important new solution in the fight against PAD, expand our portfolio and enable us to better serve our customers and the patients they treat.”

