The agency in 2023 tagged 61 medical device recalls as Class I — the most serious level. For comparison, the FDA recalled 60 devices in 2022, of which four were related to catheter-based devices.
Last year, the most serious catheter-based device recalls included products from major companies, including Medtronic and Abbott, and catheters ranging from contract material delivery to hemodialysis catheters.
Here are the seven most serious catheter-based device recalls of 2023.
Cordis: Infiniti Angiographic Catheter
Number of devices recalled: 30
Cordis’s recall of some Infiniti angiographic catheters due to some products being shipped to end users without undergoing sterilization procedures was labeled as Class I by the FDA. The catheters are intended to be shipped sterile directly from the manufacturer to a third-party distributor, which ships the product to end users.
The use of unsterilized angiographic catheters may result in serious acquired infections, sepsis, and, in extreme cases, death. However, no reports of death or injury are associated with the issue, according to the FDA warning letter dated December 5, 2023.
Cordis’ Infiniti angiographic catheters are used to deliver contrast dye to specific places in the blood vessels to help doctors see the blood vessels in the brain, organs or limbs during certain procedures. The catheter can be put into the body through the skin and used in multiple places simultaneously.

Abiomed: Impella RP Flex with SmartAssist
Number of devices recalled: 65
Abiomed, which is part of Johnson & Johnson, issued a voluntary correction of its Impella RP Flex with SmartAssist because the catheters’ instructions for use (IFU) did not appropriately address precautions for healthcare providers to take when treating patients whose anticoagulation clotting time is below the recommended value. The FDA labeled this recall as Class I.
The use of the affected catheters could result in serious adverse health consequences, including the risk of blood clots or particle deposits forming or death. There have been 12 reported injuries and no reports of death, according to the FDA warning letter dated August 17, 2023.
Abiomed’s Impella RP Flex with Smart Assist System Catheter is used for up to 14 days in patients with acute right heart failure after left ventricular assist device implantation. It is placed through the internal jugular vein and supports the heart’s right chamber by pumping blood into the pulmonary artery.

Abiomed: Impella 5.5 with SmartAssist
Number of devices recalled: 466
Johnson & Johnson’s Abiomed was involved in another of the serious catheter-based device recalls in 2023. It issued a recall of its Impella 5.5 with SmartAssist after receiving customer complaints about purge fluid leaking from the purge sidearm of the pump. The system will experience low purge pressures if a purge leak occurs, which can prompt alarms and require elevation. The FDA labeled the recall as Class I.
If the leak issue is not resolved, persistent low purge pressure and purge flow could lead to pump stop and loss of therapy. Failure of the pump’s support could lead to further deterioration and worsening of critical condition and may lead to serious injury or death. There have been 179 complaints, three injuries and no reports of death, according to the FDA warning letter dated June 5, 2023.
Abiomed’s Impella 5.5 with SmartAssist System is used for up to 14 days to support the pumping chambers of the heart when there is an ongoing cariogenic shock that occurs less than 48 hours after a severe heart attack, open-heart surgery or when the heart is not functioning well due to cardiomyopathy.
Abbott: Amplatzer Steerable Delivery Sheath
Number of devices recalled: 672
Abbott’s recall involving its Amplatzer steerable delivery sheath was Class I, the most serious level. The company issued the recall because there is an increased risk of introducing air bubbles (air emboli) into patients who have procedures with this device, according to the FDA warning dated July 26, 2023.
An air embolism can lead to injuries like sudden reduction in blood flow to the heart, fast or slow heartbeat, low blood pressure and lack of enough oxygen in the blood. Abbott has reported 26 incidents and no deaths related to this issue, according to the FDA.
The Amplatzer steerable delivery sheath is a cardiac catheter inserted through the skin that provides a pathway for catheter-based devices to be introduced into the heart chambers. It is used to deliver the Amplatzer Amulet Left Atrial Appendage Occluder.
Teleflex & Arrow Intl: Pressure Injectable Central Venous Catheters
Number of devices recalled: 1,905
Teleflex recalled some of its Pressure Injectable Central Venous Catheters kits due to mislabeling regarding the presence of chlorhexidine in these products. According to the FDA warning letter dated November 6, 2023, the product code and product name are incorrectly listed as non-coated on the lids of the affected Pressure Injectable Catheter Kits. However, the banner card correctly lists the product code and name as chlorohexidine coated.
According to the warning letter, serious adverse health effects could occur in people who inadvertently use these kits. Teleflex has reported 16 incidents associated with this issue and no reports of injuries or deaths related to this issue.
The Pressure Injectable Catheter kits allow healthcare providers to access a patient’s central cardiovascular system that connects the heart and veins.
 Medtronic: Mahurkar Acute Dual Lumen High Flow (13.5 French) Hemodialysis Catheters (Mahurkar QPlus)
Medtronic: Mahurkar Acute Dual Lumen High Flow (13.5 French) Hemodialysis Catheters (Mahurkar QPlus)
Number of devices recalled: 22,763
The FDA labeled Medtronic’s recall of some Mahurkar hemodialysis catheters as Class I, the most serious kind. Medtronic recalled the Mahurkar acute dual lumen high flow 13.5 Fr hemodialysis catheters, also known as Mahurkar QPlus, due to reports of a potential catheter hub defect that could cause leaks in a catheter’s tubes.
The leak could result in arterial and venous blood mixing during treatment, leading to increased recirculation and poor dialysis. The defect could also cause the development of blood clots in the blood vessels. Continued use of the catheters could result in serious adverse health outcomes, including bleeding or the need for surgical removal and replacement of the affected catheter. Two reports of injuries related to this issue have been reported and no deaths, according to the FDA warning letter.
Mahurkar Acute Dual Lumen High Flow (13.5 French) Hemodialysis Catheters are implanted and used in hemodialysis, aphaeresis and infusion.
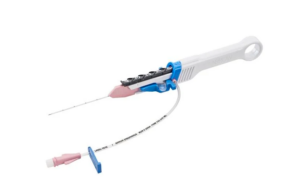
Teleflex & Arrow Intl: Arrow Endurance Extended Dwell Peripheral Catheter System
Number of devices recalled: 262,016
The FDA designated Teleflex’s recall of its Arrow Endurance extended dwell peripheral catheter system as Class I, the most serious type of medical device recall. According to the June 26, 2023 FDA warning letter, Teleflex initiated the recall after receiving catheter separation or leakage reports.
If the catheter separates while in a blood vessel, the catheter fragments could be left in the bloodstream and may migrate to other places in the body. The situation could cause a person to experience a blockage of blood vessels, inadequate blood flow, injury to blood vessel walls, blood clots, blockage of the lung arteries (pulmonary embolism), heart attack, or death, according to the FDA. Teleflex and its Arrow International subsidiary have reported 83 complaints about this issue. There are reports of 18 injuries but no deaths.
The Endurance system provides short-term access to a person’s peripheral vascular system, enabling health providers to sample blood, monitor blood pressure, or administer fluids, blood, and blood products.
