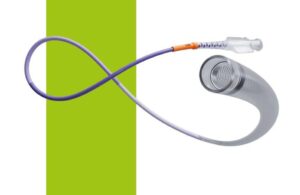
The catheter’s indication covers the revascularization of patients suffering from acute ischemic stroke. It joins the company’s planned CereGlide family of catheters set to enhance the Cerenovus Stroke Solutions portfolio. The company said it optimized the system for effective direct aspiration and for the delivery of compatible stent retrievers. That includes the EmboTrap III revascularization device into the neurovasculature.
Related: Device design and development tips for the future of stroke care
Cerenovus equipped the system with TruCourse technology to increase the flexibility of the device. It designed it to help physicians with improved navigation and access to clots, even in challenging anatomical conditions. The company said it provides optimal compatibility, durable delivery and reliable traceability during thrombectomy procedures.
In a news release, Dr. Fawaz Al-Mufti, the first global user, touted the system’s navigation, access to occlusion sites and engagement with clots. The interventional neurologist in New York said it helps to rapidly restore blood flow and provide a “potentially life-saving intervention.”
Cerenovus plans to include CereGlide 71 — now commercially available in — in the U.S. in the next phase of a real-world registry. The CERENOVUS EXCELLENT Registry focuses on stroke-inducing blood clot removal by mechanical thrombectomy.
“CereGlide 71 intermediate catheter is specifically designed to glide through challenging anatomical conditions,” said Mark Dickinson, worldwide president, Cerenovus. “Developed through robust research and clinical insights, our team designed a catheter for physicians that addresses unmet clinical needs by providing reliable trackability, durable delivery and the versatility for both direct aspiration and stent-retriever use – even in the most challenging anatomical conditions.”
Medical Design & Outsourcing: Cerenovus President Mark Dickinson on the future of stroke care

