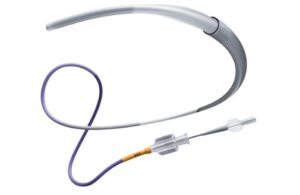
Johnson & Johnson MedTech [WtwhTicker symbol="JNJ"](NYSE: JNJ)[/WtwhTicker] announced today that it launched the CereGlide 92 catheter system for treating acute ischemic stroke. The launch comes just one day after media reports suggested that the company wants to sell its Cerenovus stroke business, the unit that makes the CereGlide … [Read more...] about Johnson & Johnson MedTech launches catheter system for stroke
Cerenovus
FDA says recall of Cerenovus catheter guide sheaths is serious
The FDA deemed a recall of catheter guide sheaths from Johnson & Johnson’s (NYSE:JNJ) Cerenovus Class I, the most serious kind. Medos International Sàrl, a J&J unit, recalled the Cerenovus Cerebase DA guide sheath and single-use neurovascular guide catheter on Feb. 2, 2024. The company distributed the devices — 1,343 in total — between … [Read more...] about FDA says recall of Cerenovus catheter guide sheaths is serious
J&J’s Cerenovus launches next-gen stroke revascularization catheter
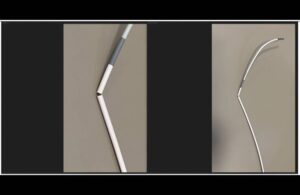
Johnson & Johnson MedTech's Cerenovus today announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse. The catheter's indication covers the revascularization of patients suffering from acute ischemic stroke. It joins the company's planned CereGlide family of catheters set to enhance the Cerenovus Stroke … [Read more...] about J&J’s Cerenovus launches next-gen stroke revascularization catheter
Cerenovus President Mark Dickinson on the future of stroke care
Cerenovus President Mark Dickinson forecasts the innovative technologies that will advance stroke care in the coming years. It's getting harder to beat aspiration systems for fast and simple thrombectomies to remove blood clots that are blocking oxygen from a stroke patient's brain. That's according to Cerenovus Worldwide President Mark … [Read more...] about Cerenovus President Mark Dickinson on the future of stroke care
Johnson & Johnson’s Cerenovus launches new clot removal devices to treat stroke
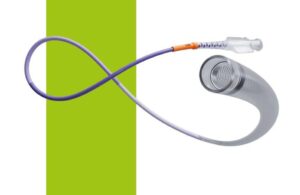
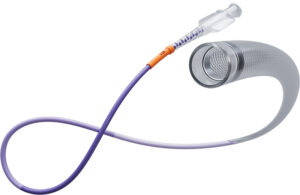
Johnson & Johnson's Cerenovus recently announced it has launched its new stroke solutions platform that includes three devices for clot removal procedures. The three devices are designed to aid physicians in performing mechanical thrombectomy procedures. The devices include the Cerebase DA Guide Sheath, Cerenovus Large Bore Catheter and … [Read more...] about Johnson & Johnson’s Cerenovus launches new clot removal devices to treat stroke