The single-shot mapping and ablation catheter uses pulsed field energy to treat patients with paroxysmal AFib. Medtronic presented interim findings on the system at the European Heart Rhythm Association (EHRA) 2024 Annual Meeting.
Designed with the goal to simplify the AFib procedure while enhancing efficiency and providing high lesion durability, the Sphere-360 catheter features a large, tissue-conformable lattice tip for energy delivery. It requires no need to rotate the catheter multiple times in one location as the entire lattice tip delivers PF energy.
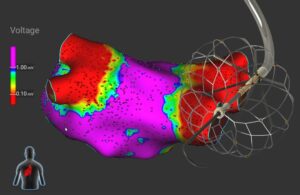
Medtronic also integrated the technology with its Affera mapping and ablation system. The Affera technology makes up a key component of Medtronic’s PulseSelect PFA system, which became the first such system FDA-cleared for treating AFib late last year. Integration offers complete visualization inside the heart and electroanatomical mapping.
According to a news release, the catheter provides a single-shot, all-in-one option for mapping, ablation and validation. The catheter features uniform and efficient PF energy delivery through the entire 34mm conformable lattice tip. Its integration with Affera technology enables low- to zero-fluoroscopy procedures.
Medtronic says its system seamlessly adjusts to various shapes to accommodate different pulmonary vein anatomies. The technology offers real-time local impedance information to assess catheter proximity to tissue. Its over-the-wire design helps with ease of positioning in the vein and streamlined workflow. The system also has compatibility with a small 8.5Fr sheath.
A look at the Medtronic study results
Medtronic’s Sphere-360 study took place across three European centers, evaluating 85 patients in total. It demonstrated 81.8% freedom from atrial arrhythmias after one year of follow-up. Medtronic also reported 100% freedom in a sub-group treated with the optimized and most recent pulse configuration. The study recorded treatment time — first to last PF application — of 10 minutes on average.
A sub-study treated patients undergoing remapping procedures with the optimized pulse. In that study, 96% of patients demonstrated sustained lesion durability. Among all pulmonary veins treated in the group, 99% remained isolated after 75 days of the index procedure.
Medtronic also reported a highly favorable safety profile with zero incidences of primary safety adverse events. That includes esophageal events, pulmonary vein stenosis, phrenic nerve injury or cardiac tamponade.
Dr. Vivek Reddy, director of cardiac arrhythmia services at the Icahn School of Medicine at Mount Sinai served as primary investigator. Reddy said the results showed Sphere-360’s potential to become “an important part of next-generation AFib care.”
“We have a vision to continually innovate and bring the best technology to AFib patients around the world, driven by our unwavering commitment to maximizing the safety and efficacy of our products,” said Dr. Khaldoun Tarakji, VP and chief medical officer of Medtronic Cardiac Ablation Solutions. “We are thrilled that the Sphere-360 study results validate our dedication to this goal: an all-in-one single shot mapping and pulsed field ablation catheter, that is fully integrated with the Affera mapping and ablation system, with a unique design that can conform to any pulmonary vein anatomy and can be used with an 8.5Fr sheath, single transeptal access and zero exchange. All these features make the procedure efficient and outcomes more predictable. We can’t wait for what’s next.”

