Olympus announced today that it closed its acquisition of Taewoong Medical, a Korea-based medical device manufacturer. Taewoong Medical develops gastrointestinal (GI) metallic stents, among other offerings. Olympus announced its intent to acquire the company for $370 million in February 2023. The deal includes $255.5 million upfront, with up to … [Read more...] about Olympus completes acquisition of GI stent maker Taewoong Medical
olympus
Olympus bronchoscopes in Class I recall could lead to burns, fire
The FDA labeled a recall of Olympus bronchoscopes that could lead to burns or fire Class I, the most serious kind. Olympus reports 192 complaints related to the issue, including four injuries. The company has received no reports of death. This recall — a correction, not a product removal — applies to Olympus bronchofiberscopes and … [Read more...] about Olympus bronchoscopes in Class I recall could lead to burns, fire
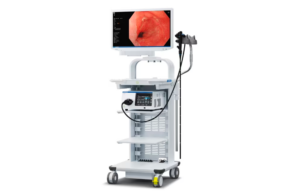
Olympus launches Evis X1 endoscopy system
Olympus recently announced it launched the Evis X1 endoscopy system in the U.S. The Evis X1 received FDA clearance earlier this year with two compatible endoscopes, the GIF-1100 gastrointestinal videoscope and the CF-HQ1100DL/I colonovideoscope. It is indicated for use in the upper digestive tract and the lower digestive tract. Evis X1 has an … [Read more...] about Olympus launches Evis X1 endoscopy system
Olympus corrective action covers laser-compatible bronchoscopes
Olympus is warning health providers about incompatible laser use with bronchoscopes after receiving reports of endobronchial combustion, serious patient injury, and one death. The Japanese medtech giant said on July 3 that its voluntary field corrective action involves 32 BF series endoscope models, 19 of which it distributes in the U.S. The … [Read more...] about Olympus corrective action covers laser-compatible bronchoscopes
Olympus to establish series of digital centers based on Odin Vision’s cloud-AI tech
Olympus announced that it plans to establish a series of digital excellence centers (DECs) following its acquisition of Odin Vision. London-based Odin Vision develops a portfolio of commercially available computer-aided detection/diagnostic (CAD) solutions. It also holds an innovation pipeline of cloud-enabled applications. Olympus agreed to … [Read more...] about Olympus to establish series of digital centers based on Odin Vision’s cloud-AI tech
FDA clears Olympus endoscopy systems and endoscopes
Olympus today announced it received FDA clearance for its new Evis X1 endoscopy system and two compatible gastrointestinal endoscopes. The FDA clearance covers Olympus’ GIF-1100 gastrointestinal videoscope indicated for use in the upper digestive tract, and the CF-HQ1100DL/I colonovideoscope indicated for use in the lower digestive … [Read more...] about FDA clears Olympus endoscopy systems and endoscopes
FDA sends warning letter to Olympus over endoscope manufacturing
The FDA issued Olympus a warning letter regarding adultered devices following an inspection of the company’s Tokyo facility. This marks the latest warning letter sent by the FDA to Olympus. The company received two separate letters at the end of last year. According to the letter, the agency conducted an inspection of the Tokyo facility … [Read more...] about FDA sends warning letter to Olympus over endoscope manufacturing
Olympus acquires GI stent maker Taewoong Medical for $370M
Olympus announced that it agreed to acquire Korea-based Taewoong Medical, a maker of medical devices, including stents, for $370 million. Taewoong Medical manufactures devices including gastrointestinal (GI) metallic stents. Olympus intends to use the acquisition to strengthen its GI endotherapy portfolio capabilities. Get the full story at our … [Read more...] about Olympus acquires GI stent maker Taewoong Medical for $370M
FDA hits Olympus with warning letters after factory inspections
The FDA today released warning letters against Olympus Medical Systems Corp. and Olympus Corp. subsidiary Aizu Olympus following inspections of their endoscope and reprocessor manufacturing facilities. The warning letters allege medical device reporting (MDR) and quality system violations at the manufacturing operations. “Olympus’ highest … [Read more...] about FDA hits Olympus with warning letters after factory inspections
The top 10 catheter innovations of 2021
Last year was a big year for catheter innovation as medtech companies large and small received regulatory approvals for devices ranging from TAVR to single-use endoscopes. Catheter innovations weren't limited to just adults; Catheter-deployed devices for premature babies and toddlers were also approved and released. And catheter-based devices … [Read more...] about The top 10 catheter innovations of 2021