FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts
stents
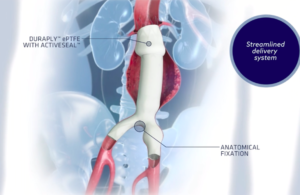
Cook Medical wins FDA breakthrough device designation for next-gen endovascular graft
Cook Medical announced that FDA has granted breakthrough device designation for its Zenith Fenestrated+ endovascular graft (ZFEN+), the next-gen version of its Zenith Fenestrated AAA endovascular graft. The designation — a first for Bloomington, Ind.–based Cook Medical — will enable priority review and better communication with FDA during the … [Read more...] about Cook Medical wins FDA breakthrough device designation for next-gen endovascular graft
Passing the test with vascular stents
Pulsatile durability testing is a time-consuming part of the vascular stent approval process. Accelerated test designs can deliver a high throughput when every specimen counts. Pete Bailey, Instron There are many quality controls applied to the design and release of coronary and other vascular stent devices, but a proof test of pulsatile … [Read more...] about Passing the test with vascular stents