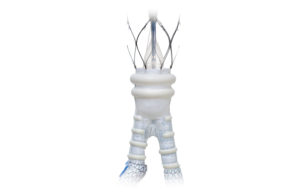
Endologix (NSDQ:ELGX) today said it launched its Alto abdominal stent graft in Canada following its recent Health Canada approval.
The Alto abdominal stent graft system is a polymer-based therapy for abdominal aortic aneurysm (AAA) patients. It uses a low-profile delivery system with a conformable sealing ring that molds in-situ to the patient’s specific aortic neck anatomy.
“We are excited to extend the global reach of Alto, which is now available in Europe, New Zealand, Canada and Argentina, as well as the U.S.,” chief medical officer Matt Thompson said in a news release. “Alto fulfills an unmet clinical need, offering a highly differentiated endovascular treatment option for patients with AAA and offers the ability to treat the largest proportion of patients within the approved indications for use.”
The first Alto stent graft implant procedure was performed at Hôpital Saint-François-d’Assise in Quebec for a patient within the approved indications for use despite the presence of hostile neck anatomy and narrow access vessels, according to Endologix.
“As an early adopter of polymer technology, I have seen how Alto’s custom sealing technology provides a precise patient-specific seal for a diverse range of anatomies,” Ghislain Nourissat, who performed the first procedure in Canada, said. “With this technology, as well as its low profile and short neck indication, I believe Alto has the potential to become the standard in endovascular aneurysm repair.”
