 Endologix announced today that the FDA granted breakthrough device designation for its Chimney endovascular aneurysm sealing system (Chevas).
Endologix announced today that the FDA granted breakthrough device designation for its Chimney endovascular aneurysm sealing system (Chevas).
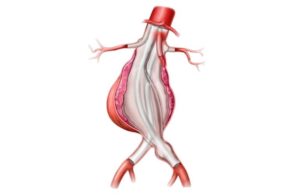
Irvine, Calif.–based Endologix designed its Chevas system as an investigational endovascular abdominal aortic aneurysm (AAA) sealing therapy that combines the Nellix 3.5 endograft with parallel visceral stents to treat patients with juxtarenal, pararenal and suprarenal AAA, according to a news release.
