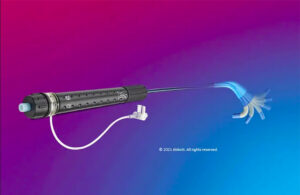
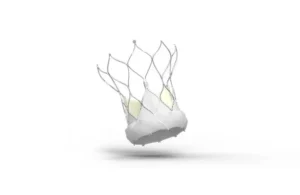
The FDA today said an Abbott recall involving its Amplatzer steerable delivery sheath is Class I, its most serious level. Launched in the U.S. in April 2022, the Amplatzer sheath delivers Abbott's Amplatzer Amulet left atrial appendage occluder. Competing against Boston Scientific's Watchman, Amplatzer Amulet provides complete occlusion in order … [Read more...] about Abbott has a serious recall involving its Amplatzer delivery sheath
Abbott
FDA approves Surmodics SurVeil drug-coated balloon
Surmodics (Nasdaq:SRDX) announced today that the FDA granted approval for its SurVeil drug-coated balloon (DCB). Eden Prairie, Minnesota–based Surmodics may now market and sell SurVeil in the U.S. for percutaneous transluminal angioplasty. Use follows appropriate vessel preparation or de novo or restenotic lesions in femoral and popliteal … [Read more...] about FDA approves Surmodics SurVeil drug-coated balloon
The top catheter innovation news stories of 2023 — so far
The world of catheter innovation has already seen remarkable strides this year— with some of the most promising medtech delivered into the body via catheter-based procedures. Think of improved ablation for treating AFib, coronary intravascular lithotripsy to combat coronary artery disease, and more. Here, we've highlighted the top five … [Read more...] about The top catheter innovation news stories of 2023 — so far
Abbott wins FDA approval for next-gen TactiFlex ablation catheter
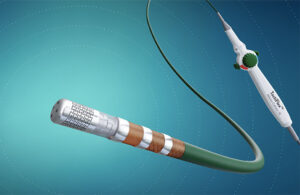
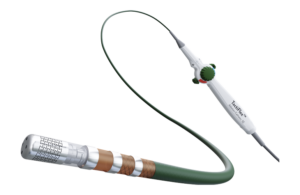
Abbott (NYSE: ABT) today announced FDA approval of its TactiFlex Ablation Catheter, Sensor Enabled, which the company describes as the world's first ablation catheter with a flexible tip and contact force technology. The TactiFlex catheter also integrates with Abbott's EnSite X EP System, enabling physicians to visualize heart anatomy more … [Read more...] about Abbott wins FDA approval for next-gen TactiFlex ablation catheter
Abbott reports positive data for TriClip TEER system
Abbott this week announced late-breaking data supporting the benefits of its TriClip transcatheter edge-to-edge repair (TEER) system. The company presented study data at EuroPCR in Paris. This study evaluated TriClip, a first-of-its-kind, minimally invasive device, in treating patients with leaky tricuspid valves. Abbott’s bRIGHT study … [Read more...] about Abbott reports positive data for TriClip TEER system
The biggest cardiology tech stories from ACC.23
Major medtech players presented a range of intriguing studies at a gathering of some of the biggest names in the cardiology tech space. Some of the hottest topics were covered this past week at the American College of Cardiology’s Annual Scientific Session Together With the World Congress of Cardiology (ACC.23/WCC) in New Orleans. Ablation, … [Read more...] about The biggest cardiology tech stories from ACC.23
Abbott scores regulatory wins in electrophysiology
Abbott today announced two new regulatory approvals related to its TactiFlex and FlexAbility ablation catheters. TactiFlex ablation catheter, sensor enabled (SE), received a CE mark for treating abnormal heart rhythms such as atrial fibrillation. The company's FlexAbility ablation catheter, SE, also received an expanded indication from the FDA … [Read more...] about Abbott scores regulatory wins in electrophysiology
Abbott wins FDA nod for its Navitor transcatheter aortic valve implant
Abbott (NYSE:ABT) announced this week that it received FDA approval for its latest-generation transcatheter aortic valve implantation (TAVI) system. The company designed the Navitor system for treating people with severe aortic stenosis at high or extreme risk for open-heart surgery. It adds to Abbott’s transcatheter structural heart … [Read more...] about Abbott wins FDA nod for its Navitor transcatheter aortic valve implant
The top 10 catheter innovation news stories of 2022
This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022
Abbott launches TactiFlex SE radiofrequency ablation catheter in Japan
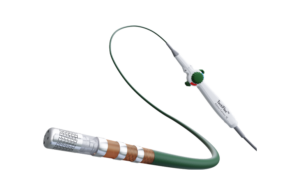
Abbott this week announced it has launched its TactiFlex SE irrigation catheter in Japan. TactiFlex SE is a radiofrequency (RF) ablation catheter with a slit tip electrode with contact force technology. The tip of the catheter is flexible and conforms to the shape of the heart's wall for unique patient anatomies. The slit structure on the … [Read more...] about Abbott launches TactiFlex SE radiofrequency ablation catheter in Japan