St. Jude Medical, now part of Abbott, has won a patent dispute over the method for using a double catheter in heart failure patients. Niazi Licensing's patent, issued in 2003 for the double catheter system, describes it as better suited for “use in the coronary sinus, especially in patients suffering from congestive heart failure,” according to … [Read more...] about Abbott’s St. Jude Medical wins patent spat over catheterization method
Abbott
Abbott touts Xience DES study results
A pair of studies of Abbott’s Xience drug-eluting stent showed no difference between shorter courses of treatment with dual antiplatelet therapy (DAPT) compared with 12 months of DAPT following implantation of the XIENCE drug-eluting stent (DES) in patients who are at a high risk of bleeding. Abbott presented the late-breaking data today at TCT … [Read more...] about Abbott touts Xience DES study results
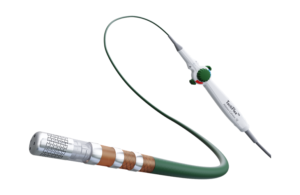
Abbott launches study of next-gen ablation catheter
Abbott (NYSE:ABT) today announced it has enrolled the first participants in its TactiFlex PAF IDE study to evaluate the performance of the TactiFlex sensor-enabled ablation catheter. Abbott designed the catheter to treat people suffering from paroxysmal atrial fibrillation (PAF) with symptoms that are unable to be managed by … [Read more...] about Abbott launches study of next-gen ablation catheter
Abbott wins CE Mark for FlexNav delivery system
Abbott (NYSE:ABT) announced today that it won CE Mark approval in Europe for the FlexNav delivery system for its Portico transcatheter aortic valve implantation (TAVI) system. The Abbott Park, Ill.-based company’s FlexNav delivery system is designed to offer another tool for doctors to treat patients requiring a transcatheter aortic valve … [Read more...] about Abbott wins CE Mark for FlexNav delivery system
Survey: Few adults know signs of heart failure
Only one in five adults can accurately recognize the signs and symptoms of heart failure, according to a new survey commissioned by Abbott (NYSE:ABT). The survey of more than 3,000 Americans revealed a significant gap in awareness of what heart failure is and an understanding of how the disease can be managed. More than six million Americans are … [Read more...] about Survey: Few adults know signs of heart failure
How innovative pump designs power intelligent balloon catheters
The next generation of intelligent balloon catheters depends on novel designs of ball-screw-driven pumps. Bruce Gretz, Steinmeyer Balloon catheters have long seen widespread use in cardiovascular applications. One of the first, decades ago, was intra-aortic. A balloon was implanted into the descending aorta and pressurized via a percutaneous … [Read more...] about How innovative pump designs power intelligent balloon catheters