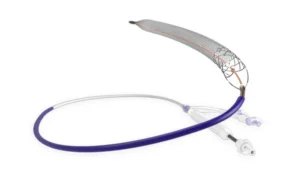
Inari Medical (Nasdaq:NARI) announced positive two-year interim results from the CLOUT registry evaluating its ClotTriever system.
Principal investigator Dr. David Dexter of Sentara Vascular Specialists in Norfolk, Virginia, presented the data at the American Venous Forum.
Results represent the largest prospective, multi-center, two-year dataset in deep-vein thrombosis (DVT) since the ATTRACT trial, Inari said in a news release. Data demonstrates the safety, effectiveness and long-term outcomes of ClotTriever in real-world DVT patients.
Inari designed ClotTriever to capture and remove large clot burden from big vessels. Treating patients in one single session, it eliminates the need for thrombolytic and a stay in the intensive care unit. The company hopes to make it the standard of care for DVT patients.
Patients showed a low incidence of independently adjudicated safety events related to thrombosis, according to the company. That metric came in at 5% and 8.4% and 30 days and six months, respectively. In the interim analysis, 228 patients completed their two-year follow-up visit. Patients had significant and sustained improvement in post-thrombotic syndrome (PTS) over the course of follow-up.
Inari reported just 7.3% of patients with moderate-severe PTS at two years. PTS rates in CLOUT provided significantly lower than historical DVT studies, which saw rates between 18% and 24%.
“This data continues to reinforce the strong safety and effectiveness profile of the ClotTriever system, which is not only the most utilized, but also the most studied thrombectomy device in DVT,” said Dr. Thomas Tu, Inari’s chief medical officer. “We are committed to generating best-in-class clinical data. This includes our currently enrolling randomized controlled trial, DEFIANCE, which will compare outcomes after ClotTriever treatment vs. anticoagulation alone. No other company is pursuing this level of research in VTE.”
