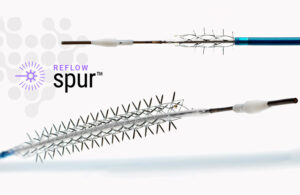
The system treats de novo or restenotic lesions in the infrapopliteal arteries, followed by a commercially available drug-coated balloon, to enhance drug absorption. The goal is to provide stent-like results while leaving no metal behind in below-the-knee lesions.
“The device performance and clinical study data for patients suffering from [chronic limb-threatening ischemia] has been quite impressive,” said Dr. Thomas Zeller, chief of the Department of Angiology at University Heart Center Freiburg in Bad Krozingen, Germany. Zeller was a principal investigator in the Deeper OUS clinical trial.
According to San Clemente, California–based Reflow Medical, the Bare Temporary Spur Stent System — followed by drug-coated balloon treatment — reduces clinically driven target lesion revascularization. In addition, it improves wound healing, reduces recoil, and improves vessel patency through one year. The company said the system compares well against historical treatment outcomes with plain balloon angioplasty or a drug-coated balloon alone.
Known as retrievable stent therapy, or RST, the self-expanding stent has radial spikes to create channels in the vessel wall to enhance drug absorption and reduce recoil. A health provider then recaptures and removes the stent before treatment with a drug-coated balloon.
“We have found that the Spur allows us to treat patients with [below-the-knee] disease using stent therapy, without the long-term risk of a stent implant,” said Dr. Marianne Brodmann, professor and vascular specialist with the Division of Angiology, Medical University Graz in Graz, Austria, and the Principal Investigator of the Deeper LIMUS clinical trial.
Isa Rizk, Reflow Medical’s co-founder and CEO, described the CE mark as a huge milestone.
“It enables us to offer a clinically validated solution to an unmet need in a major disease area,” Rizk said. “Our next goal is to expand our organization to commercialize this breakthrough technology and serve the needs of physicians and their patients in countries accepting this certification.”

