The medical device industry as a whole experienced a handful of innovations in 2021, but Medical Tubing + Extrusion's reader took a special interest in two catheter-based innovations last year. Here are our most popular articles of 2021: Links to articles: Cook Medical wins FDA breakthrough device designation for next-gen … [Read more...] about The top stories from Medical Tubing + Extrusion in 2021
Cook Medical
Cook Medical issues voluntary recall for transseptal needles, transseptal needles with catheter
Cook Medical this week announced a global voluntary recall of its Transseptal Needle and Transseptal Needle with catheter. Bloomington, Indiana-based Cook Medical issued the recall due to complaints of rust on the products. The use of both products could result in increased procedural time and inflammatory reactions, including systemic reactions … [Read more...] about Cook Medical issues voluntary recall for transseptal needles, transseptal needles with catheter
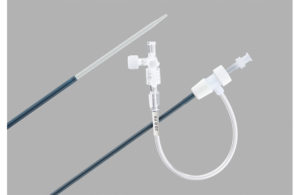
Cook Medical warns on some Flexor Check-Flo introducer sheaths
Cook Medical recently issued a warning letter regarding an issue with its Flexor Check-Flo introducer catheters. Bloomington, Indiana-based Cook Medical's field safety corrective action informs users that some of its introducer sheaths may be manufactured incorrectly and that the radiopaque marker band may be located just below the Check-Flo … [Read more...] about Cook Medical warns on some Flexor Check-Flo introducer sheaths
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts
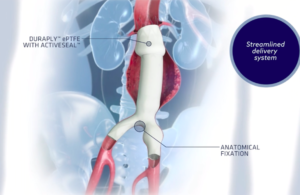
Cook Medical wins FDA breakthrough device designation for next-gen endovascular graft
Cook Medical announced that FDA has granted breakthrough device designation for its Zenith Fenestrated+ endovascular graft (ZFEN+), the next-gen version of its Zenith Fenestrated AAA endovascular graft. The designation — a first for Bloomington, Ind.–based Cook Medical — will enable priority review and better communication with FDA during the … [Read more...] about Cook Medical wins FDA breakthrough device designation for next-gen endovascular graft
The worst catheter-based device recalls of 2020
The U.S. saw nine serious medical device recalls related to catheters in 2020 — up from four the previous year, according to the FDA. The agency 2020 tagged a total of 33 medical device recalls as Class I — the most serious level — down from 49 in 2019. The list of the most serious catheter-based device recalls in 2020 includes products from … [Read more...] about The worst catheter-based device recalls of 2020
Cook Medical has a serious catheter recall
FDA has designated Cook Medical's recall of its Flexor Check-Flo introducers and Flexor tuohy-borst side-arm introducers as Class I, its most serious level. Cook Medical initiated the recall on Nov. 24. It involves 37,326 of the devices, which are used to deliver medical devices to blood vessels (though not vessels of the heart and brain). The … [Read more...] about Cook Medical has a serious catheter recall
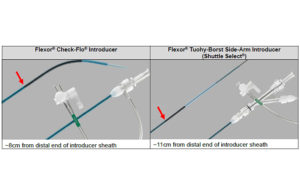
Cook Medical launches global catheter introducer recall
Cook Medical has launched a voluntary recall of nearly 59,000 devices intended to introduce therapeutic or diagnostic devices into the vasculature, excluding coronary and neuro vasculature. The Flexor Check-Flo introducer and Flexor Tuohy-Borst side-arm introducer (Shuttle Select) are sterile, single-use devices that incorporate a … [Read more...] about Cook Medical launches global catheter introducer recall
Cook Medical announces U.S. launch of balloon for transnasal esophageal procedures
Cook Medical today commercially launched its Hercules 100 Transnasal Esophageal Balloon in the U.S. The balloon is designed specifically for trans nasal esophageal procedures and is another tool for ENT physicians to help treat esophageal strictures. "This balloon was specifically designed with ENT physicians and their patients in mind. The … [Read more...] about Cook Medical announces U.S. launch of balloon for transnasal esophageal procedures