Results came out of the statistical dissection primary arm from the TRIOMPHE FDA investigational device exemption (IDE) study. Investigators presented findings at the American Association for Thoracic Surgery (AATS) Annual Meeting in Seattle.
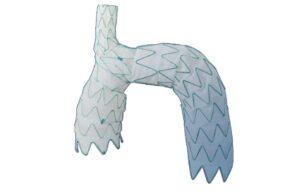
TRIOMPHE evaluates the safety and efficacy of the Nexus aortic arch stent graft for the treatment of aortic arch disease. The trial takes place across 30 aortic centers in the U.S. and one in New Zealand. Endospan completed enrollment in TRIOMPHE in October 2024.
The three-arm study assesses Nexus in patients with aortic arch pathologies, including dissection, aneurysm and PAU/IMH. Endospan says 30-day results were promising for the Nexus system and suggest that the device may be an acceptable alternative to open aortic arch replacement in select patients at high risk for open surgery.
Core lab analysis of stent graft sealing showed no Type IA, no Type IB and no Type III endoleaks. This suggests good sealing at 30 days. Principal investigator Dr. Brad Leshnower called the results “highly encouraging.” Leshnower says the low stroke rate particularly represented “a significant achievement.”
“We are very encouraged by the results of the first ever presented data of endovascular aortic arch repair in Zone 0 in a pivotal IDE study,” said Kevin Mayberry. CEO of Endospan. “We look forward to continue our leadership in clinical development of Aortic Arch repair in the future.” The TRIOMPHE study is ongoing, and longer-term follow-up will provide further insights into the safety and effectiveness of the Nexus aortic arch stent graft system.
