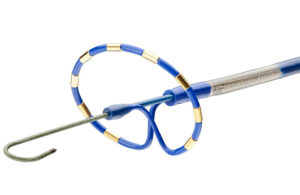
Medtronic’s PulseSelect Pulsed Field Ablation (PFA) System — which won the first FDA approval for PFA to treat atrial fibrillation (AFib) — is just the start of a wave of new PFA devices expected to hit the market. Medtronic is lining up another PFA cardiac ablation system for approval, while competitor Boston Scientific anticipates approval of … [Read more...] about What’s so special about pulsed field ablation? Medtronic EVP Sean Salmon explains
afib
Medtronic PulseSelect pulsed field ablation wins FDA approval
The FDA has approved the Medtronic PulseSelect pulsed field ablation (PFA) system, the device developer said today. PulseSelect is the first PFA technology approved for use in the U.S., as well as the first PFA technology with FDA breakthrough designation to win approval. The minimally invasive, cardiac ablation system is indicated for the … [Read more...] about Medtronic PulseSelect pulsed field ablation wins FDA approval
Biosense Webster launches HelioStar in Europe
Biosense Webster today launched its HelioStar balloon ablation catheter in Europe. Johnson & Johnson's subsidiary Biosense Webster launched the radiofrequency balloon ablation catheter for catheter-based cardiac electrophysiological mapping of the atria when used with a multi-channel RF generator. According to the company, more than 11 … [Read more...] about Biosense Webster launches HelioStar in Europe
CoreMap raises $23 million to fund AFib mapping development
CoreMap today announced it closed a $20 million Series B financing to accelerate the development of its cardiac arrhythmia diagnostic technology and launch pilot studies. The Burlington, Vermont–based company said it also closed on a $3 million Series A Milestone financing that was part of its $10.5 million Series A in September 2020. In … [Read more...] about CoreMap raises $23 million to fund AFib mapping development