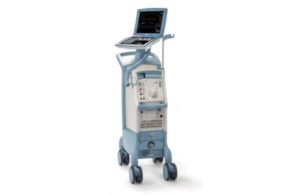
The FDA determined that Getinge's recall of its Cardiosave Hybrid and CardioSave Rescue devices is Class I, the most serious kind. Datascope/Getinge/Maquet designed the CardioSave Hybrid and CardioSave Rescue intra-aortic balloon pumps (IABP) as cardiac assist devices for patients undergoing cardiac and non-cardiac surgery. They are used in … [Read more...] about Getinge intra-aortic balloon pump recall is Class I
fda
ALung Technologies wins FDA de novo nod for Hemolung respiratory assist system
ALung Technologies announced today that it received FDA de novo clearance for its Hemolung respiratory support platform. Pittsburgh-based ALung designed its Hemolung system to provide low-flow extracorporeal carbon dioxide removal (ECCO2R) for patients with acute respiratory failure. Get the full story at our sister site, MassDevice. … [Read more...] about ALung Technologies wins FDA de novo nod for Hemolung respiratory assist system
What is renal denervation? Medtronic Coronary and RDN President Jason Weidman explains
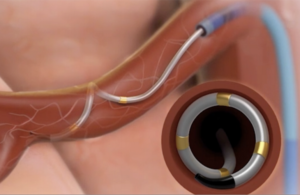
Medtronic (NYSE:MDT) continues clinical trials of its Symplicity Spyral renal denervation (RDN) system for treating hypertension. The hope now is to win FDA approval in 2023. To better understand the technology behind what Medtronic leaders expect to become a multibillion-dollar business, Medical Design & Outsourcing spoke with Jason … [Read more...] about What is renal denervation? Medtronic Coronary and RDN President Jason Weidman explains
Medtronic faces longer road to renal denervation
Medtronic (NYSE:MDT) will continue the clinical study of its Symplicity Spyral renal denervation (RDN) system for hypertension into next year after lacking the positive results needed to end enrollment early. Fridley, Minnesota-based Medtronic said last month that it hoped to present results of the Spyral HTN-ON MED trial at the Cardiovascular … [Read more...] about Medtronic faces longer road to renal denervation
Medtronic’s Pipeline Flex problems expand
The FDA today issued a notice confirming a Class I recall of the Pipeline Flex embolization device from Medtronic (NYSE:MDT). Several models of the Pipeline Flex embolization device and Pipeline Flex embolization device with Shield Technology were affected, with 8,825 devices recalled in the U.S., having been distributed between April 18, 2019, … [Read more...] about Medtronic’s Pipeline Flex problems expand
Glaukos submits supplemental PMA application for iStent Infinite
Glaukos (NYSE:GKOS) announced today that it submitted a supplemental premarket approval application to the FDA for its iStent Infinite system. San Clemente, California–based Glaukos designed the iStent Infinite trabecular micro-bypass system for use in a standalone procedure to reduce elevated intraocular pressure (IOP) in patients with … [Read more...] about Glaukos submits supplemental PMA application for iStent Infinite
Medtronic has a Class I recall of some angiographic guidewires
The FDA has classified the recall of some angiographic guidewires made by Medtronic (NYSE:MDT) as Class I, the most serious kind. Medtronic’s angiographic guidewire component affected by the recall helps place catheters into the vasculature during angiography or other interventional procedures. Get the full story at our sister site, MassDevice. … [Read more...] about Medtronic has a Class I recall of some angiographic guidewires
Abbott wins new FDA approvals for stent family including nod for next-gen Xience Skypoint
Abbott (NYSE:ABT) announced today that the Xience stent family received FDA approval for one-month dual antiplatelet therapy (DAPT) labeling. The FDA’s approval for one-month (as short as 28 days) DAPT labeling applies to high bleeding risk (HBR) patients in the U.S., according to a news release. The company also recently received CE mark … [Read more...] about Abbott wins new FDA approvals for stent family including nod for next-gen Xience Skypoint
FDA grants PMA to Abiomed’s next-generation Impella RP
Abiomed (NSDQ:ABMD) announced today that the FDA granted premarket approval (PMA) to its Impella RP with SmartAssist. Danvers, Mass.–based Abiomed received PMA for the Impella RP right heart pump with SmartAssist as a safe and effective treatment for acute right heart failure for up to 14 days, according to a news release. Get the full story at … [Read more...] about FDA grants PMA to Abiomed’s next-generation Impella RP
Medtronic wins expanded FDA approval for cryoablation catheters
Medtronic (NYSE:MDT) announced today that it received FDA expanded approval for its Arctic Front family of cardiac cryoablation catheters. Fridley, Minn.-based Medtronic's Arctic Front family of catheters treat recurrent symptomatic paroxysmal atrial fibrillation (AFib) as an alternative to antiarrhythmic drug (AAD) therapy as an initial rhythm … [Read more...] about Medtronic wins expanded FDA approval for cryoablation catheters