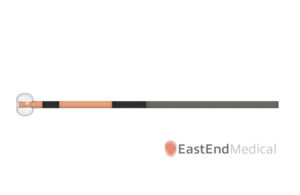
East End Medical this week announced that it received FDA 510(k) clearance for its SafeCross transseptal radiofrequency (RF) puncture and steerable balloon introducer system. The Miami-based company designed SafeCross to provide a predictable and safe treatment option for electrophysiology and structural heart interventions requiring left atrial … [Read more...] about East End Medical wins FDA clearance for transseptal balloon introducer
Balloons
FDA clears Front Line Medical arterial occlusion system
Front Line Medical Technologies today announced that its COBRA-OS bleeding control device won FDA 510(k) clearance. The COBRA-OS (control of bleeding, resuscitation, arterial occlusion system) is a 4 French REBOA (resuscitative endovascular balloon occlusion of the aorta) device that provides full occlusion, intermittent occlusion or partial … [Read more...] about FDA clears Front Line Medical arterial occlusion system
Cardiovascular Systems launches peripheral balloon catheter
Cardiovascular Systems (NSDQ:CSII) today said it has launched its OrbusNeich Jade percutaneous transluminal angioplasty over-the-wire balloon catheter in the U.S. Jade is an over-the-wire (OTW) balloon catheter for the peripheral vasculature, including obstructed native arteries and synthetic arteriovenous dialysis fistulae and post-dilation of … [Read more...] about Cardiovascular Systems launches peripheral balloon catheter
Boston Scientific initiates coronary drug-coated balloon study in U.S.
Boston Scientific (NYSE:BSX) this week launched its Agent IDE trial for its Agent drug-coated balloon. The U.S. prospective, randomized clinical trial will evaluate the safety and effectiveness of a drug-coated balloon (DCB) in patients with coronary in-stent restenosis in lesions up to 26 mm in length in a coronary artery 2.0 mm to 4.0 mm in … [Read more...] about Boston Scientific initiates coronary drug-coated balloon study in U.S.
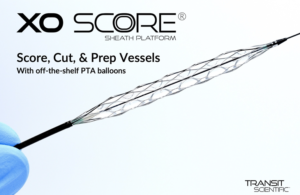
Transit Scientific scoring sheath wins CE mark
Transit Scientific today said it received CE mark clearance for its XO Score scoring sheath platform. XO Score is designed for dilation of stenotic material in the peripheral vasculature, including popliteal, infrapopliteal and native or synthetic arteriovenous dialysis fistulae. The scoring and cutting technology in the device enables … [Read more...] about Transit Scientific scoring sheath wins CE mark