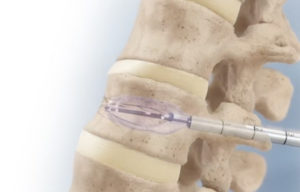
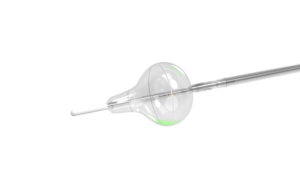
Medtronic (NYSE: MDT) says a study shows its balloon kyphoplasty (BKP) and vertebroplasty (VP) technology could eliminate opioid use among many with spinal fractures. The medical device giant sponsored a retrospective claims analysis of a large, nationally representative insurance claims database. The results — recently published in Osteoporosis … [Read more...] about Medtronic says study results show alternative spine procedures could reduce opioid use
Balloons
FDA approves Urotronic’s Optilume drug coated balloon for urethral strictures
Urotronic today announced that it received FDA approval for its Optilume urethral drug-coated balloon for male urethral strictures. Plymouth, Minnesota–based Urotronic designed the paclitaxel-coated balloon to inhibit new scar tissue growth that can occur after endoscopic dilations. Urethral strictures are scars in or around the urethra that can … [Read more...] about FDA approves Urotronic’s Optilume drug coated balloon for urethral strictures
RenovoRx wins FDA clearance for RenovoCath delivery system
RenovoRx today said it received FDA 510(k) clearance for its RenovoCath delivery system. Los Altos, California-based RenovoRx designed the RenovoCath system as the device component for its RenovoGem chemotherapy drug. The combined drug/device RenovoTAMP trans-arterial micro-perfusion therapy is a dual-balloon infusion catheter that delivers … [Read more...] about RenovoRx wins FDA clearance for RenovoCath delivery system
3 pitfalls to consider when creating catheter delivery systems
Multiple components go into a catheter delivery system, with several ways to combine them. An Edwards Lifesciences senior R&D director goes over potential pitfalls. A catheter is a tube that can deliver devices or pharmaceuticals into the body. It is also commonly used for diagnostic purposes. A catheter has a hub or a handle on its end … [Read more...] about 3 pitfalls to consider when creating catheter delivery systems
Design considerations you need to know when making ablation catheters to treat AFib
Catheters are increasingly deploying heart devices in minimally invasive procedures. CardioFocus VP of engineering Jerry Melsky describes the components and design considerations that go into making successful ablation catheters to treat AFib. Atrial fibrillation (AFib) is the most common irregular heart rhythm disorder. If the atria of the … [Read more...] about Design considerations you need to know when making ablation catheters to treat AFib
MedAlliance enrolls first patient in drug-eluting balloon trial for erectile dysfunction
MedAlliance announced today that it enrolled the first patient in an erectile dysfunction (ED) feasibility study with its drug-eluting balloon (DEB). Nyon, Switzerland-based MedAlliance’s first patient was enrolled in the trial at the University of Rome Tor Vergata, Italy, under the direction of principal investigator Giuseppe Sangiorgi, a … [Read more...] about MedAlliance enrolls first patient in drug-eluting balloon trial for erectile dysfunction
Intersect ENT launches VenSure balloon sinus dilation system in U.S.
Intersect ENT today announced that it has commercially launched its VenSure balloon sinus dilation system and Cube 4D navigation system in the U.S. Menlo Park, Calif.-based Intersect ENT designed the VenSure balloon and Cube 4D navigation system to be used in procedures that improve debilitating chronic rhinosinusitis (CRS) symptoms. Cube 4D has … [Read more...] about Intersect ENT launches VenSure balloon sinus dilation system in U.S.
Medtronic launches Prevail drug-coated balloon catheter in Europe
Medtronic today announced that it has launched its Prevail drug-coated balloon catheter in Europe. Dublin-based Medtronic designed the Prevail catheter for percutaneous coronary intervention procedures to treat narrowed or blocked coronary arteries in patients with coronary artery disease. During the procedure, the balloon inflates within the … [Read more...] about Medtronic launches Prevail drug-coated balloon catheter in Europe
The 18 most innovative medical devices of 2021
The Galien Foundation today announced the nominees for most innovative medical devices for its 15th annual Prix Galien USA Awards. The foundation nominates devices, biotechnology and pharmaceutical products for its annual Prix Galien awards to highlight products designed to improve the human condition. “The Awards Committee is excited to … [Read more...] about The 18 most innovative medical devices of 2021
FDA clears Stryker biodegradable subacromial balloon spacer
Stryker (NYSE:SYK) this week announced that it received FDA de novo clearance for its balloon implant for arthroscopic treatment of massive irreparable rotator cuff tears. Kalamazoo, Mich.–based Stryker designed the InSpace balloon implant to restore the subacromial space without requiring sutures or fixation devices. The device has demonstrated … [Read more...] about FDA clears Stryker biodegradable subacromial balloon spacer