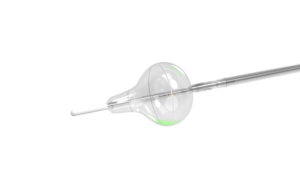
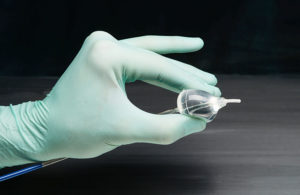
Catheters are increasingly deploying heart devices in minimally invasive procedures. CardioFocus VP of engineering Jerry Melsky describes the components and design considerations that go into making successful ablation catheters to treat AFib. Atrial fibrillation (AFib) is the most common irregular heart rhythm disorder. If the atria of the … [Read more...] about Design considerations you need to know when making ablation catheters to treat AFib
Cardiofocus
CardioFocus names new CFO
CardioFocus today said it appointed Stephan Ogilvie as chief financial officer. Prior to joining the Marlborough, Mass.-based company, Ogilvie served as managing director of healthcare investment banking at B Riley Securities. He has also served as VP of corporate development at Nuvasive and has held numerous leadership roles at ThinkEquity … [Read more...] about CardioFocus names new CFO
CardioFocus wins Japan regulatory approval for HeartLight X3 system
CardioFocus today said its HeartLight X3 catheter ablation technology received approval from the Japanese Ministry of Health, Labor and Welfare. Marlborough, Mass.-based CardioFocus designed the catheter ablation technology for controlled and consistent pulmonary vein isolation to treat atrial fibrillation (AFib). "MHLW's regulatory approval … [Read more...] about CardioFocus wins Japan regulatory approval for HeartLight X3 system
CardioFocus, China Grand Pharmaceutical partner to commercialize catheter ablation system in China
CardioFocus today announced a licensing and distribution agreement with China Grand Pharmaceutical to seek regulatory approvals for the HeartLight X3 catheter ablation technology in China. Marlborough, Mass.–based CardioFocus designed the HeartLight X3 system for controlled and consistent pulmonary vein isolation, which is the primary treatment … [Read more...] about CardioFocus, China Grand Pharmaceutical partner to commercialize catheter ablation system in China
CardioFocus seeks PMA supplement to launch HeartLight X3 endoscopic ablation system
CardioFocus is seeking a PMA supplement with FDA so that it can launch its next-gen HeartLight X3 endoscopic ablation system in the U.S. The move comes more than 10 months after Marlborough, Mass.–based won a CE Mark in Europe for the device, which is used to treat AFib. CardioFocus plans to launch HeartLight X3 in the U.S. immediately after … [Read more...] about CardioFocus seeks PMA supplement to launch HeartLight X3 endoscopic ablation system