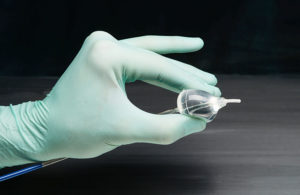
The move comes more than 10 months after Marlborough, Mass.–based won a CE Mark in Europe for the device, which is used to treat AFib. CardioFocus plans to launch HeartLight X3 in the U.S. immediately after receiving the PMA supplement, the company announced yesterday.
This supplement follows the original, approved PMA of the company’s HeartLight endoscopic system.

