Advanced NanoTherapies announced today that it collected a $4 million Series A extension from an undisclosed strategic investor. Los Gatos, California-based Advanced NanoTherapies received the investment from a medical device company. It also announced the successful treatment of the first cohort of study participants in its drug-coated balloon … [Read more...] about Advanced NanoTherapies picks up $4M investment for drug-coated balloon development
Balloons
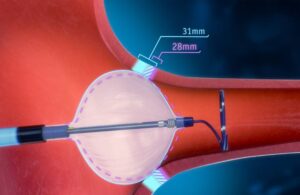
FDA approves POLARx cryoablation system from Boston Scientific
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] announced today that it received FDA approval for its POLARx cryoablation system. The new system received an indication for the treatment of patients with paroxysmal AFib. It features the POLARx FIT cryoablation balloon catheter. This device enables two balloon sizes (28 mm and … [Read more...] about FDA approves POLARx cryoablation system from Boston Scientific
Orchestra BioMed wins FDA IDE for drug-coated balloon study
Orchestra BioMed (Nasdaq:OBIO) announced today that the FDA granted investigational device exemption (IDE) for its Virtue SAB. The IDE enables the Virtue ISR-US pivotal study. It evaluates Virtue SAB, a novel AngioInfusion balloon for treating artery disease, in treating patients with coronary ISR. New Hope, Pennslyvania-based Orchestra … [Read more...] about Orchestra BioMed wins FDA IDE for drug-coated balloon study
Teleflex completes enrollment in percutaneous coronary intervention study
Teleflex (NYSE: TFX) announced today that it completed patient enrollment in its prospective multicenter Ringer PTCA study. Ringer PTCA is one of two clinical studies evaluating Teleflex's novel Ringer perfusion balloon catheter. The trial enrolled patients across seven leading complex percutaneous coronary intervention (PCI) centers in the U.S. … [Read more...] about Teleflex completes enrollment in percutaneous coronary intervention study
FDA approves drug-coated balloon for BPH symptoms from Urotronic
Urotronic announced that the FDA approved its Optilume BPH catheter system for alleviating urinary symptoms caused by BPH. Minneapolis-based Urotronic designed Optilume as a minimally invasive surgical therapy. It combines mechanical dilation using a proprietary double-lobe balloon with concurrent localized delivery of paclitaxel. This treats … [Read more...] about FDA approves drug-coated balloon for BPH symptoms from Urotronic
FDA updates guidance on paclitaxel-coated devices, determines no link to late mortality risk
The FDA issued healthcare providers updated guidance for certain warning language with paclitaxel-coated devices that treat PAD. These peripheral arterial disease (PAD)-treating devices produced data that does not support an excess mortality risk. Specifically, the FDA's guidance eliminates the need for certain warning language in the device … [Read more...] about FDA updates guidance on paclitaxel-coated devices, determines no link to late mortality risk
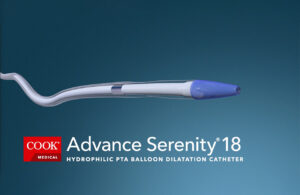
Cook Medical has more hydrophilic PTA balloon catheter options
Cook Medical today announced more sizes and locations for the Advance Serenity hydrophilic PTA balloon catheter product line. It's now possible for U.S. and Canadian interventionalists to use Advance Serenity for below-the-knee and above-the-knee procedures to treat patients with peripheral artery disease (PAD), according to Cook Medical. In … [Read more...] about Cook Medical has more hydrophilic PTA balloon catheter options
FDA approves Surmodics SurVeil drug-coated balloon
Surmodics (Nasdaq:SRDX) announced today that the FDA granted approval for its SurVeil drug-coated balloon (DCB). Eden Prairie, Minnesota–based Surmodics may now market and sell SurVeil in the U.S. for percutaneous transluminal angioplasty. Use follows appropriate vessel preparation or de novo or restenotic lesions in femoral and popliteal … [Read more...] about FDA approves Surmodics SurVeil drug-coated balloon
FDA clears Pounce LP thrombectomy system from Surmodics
Surmodics (Nasdaq:SRDX) today announced that it received FDA 510(k) clearance for its Pounce low-profile (LP) thrombectomy system. News of the regulatory nod comes just one day after Needham analysts upgraded Surmodics stock from "Hold" to "Buy." The analysts cited positive developments pointing toward the likely approval of Surmodics' SurVeil … [Read more...] about FDA clears Pounce LP thrombectomy system from Surmodics
How Medtronic uses nitinol to improve the structure and effectiveness of heart devices
Tim Laske, VP of research and business development for Medtronic‘s cardiac ablation solutions business, discusses the challenges of designing devices for the heart and explores the properties of nitinol. The heart is often one of the most underappreciated aspects of human anatomy, and its atrial appendages are often overlooked even in cardiac … [Read more...] about How Medtronic uses nitinol to improve the structure and effectiveness of heart devices