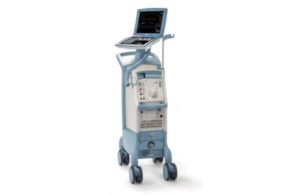
The FDA issued a notice determining another recall of Getinge subsidiary Datascope’s Cardiosave intra-aortic balloon pumps (IABPs) as Class I, the most serious kind. This recall relates to the Swedish medtech company’s Cardiosave Hybrid and Cardiosave Rescue IABPs. Both devices were subject to a separate Class I recall earlier this month. The … [Read more...] about Getinge’s Datascope has its second Class I recall in a month
Balloons
Surmodics targets FDA premarket approval for drug-coated balloon in Q4
Surmodics (Nasdaq:SRDX) announced today that it received formal feedback from the FDA related to its SurVeil drug-coated balloon (DCB). Eden Prairie, Minnesota-based Surmodics gave the FDA a proposed approach to submit an amended premarket approval application for SurVeil. In January, the FDA indicated that Surmodics’ SurVeil PMA application is … [Read more...] about Surmodics targets FDA premarket approval for drug-coated balloon in Q4
Getinge wins FDA PMA for iCast covered stent
Getinge recently announced its iCast covered stent received FDA premarket approval to treat patients with iliac arterial occlusive disease. The iCast covered stent is a clinically-evaluated balloon expandable polytetrafluoroethylene-covered stent. It is delivered using a catheter to hold open and support the walls of structures within the body. … [Read more...] about Getinge wins FDA PMA for iCast covered stent
Getinge’s Datascope has another serious intra-aortic balloon pump recall
The FDA issued a notice determining a recall of Getinge subsidiary Datascope's Cardiosave intra-aortic balloon pumps (IABPs) as Class I, the most serious kind. This recall relates to the Swedish medtech company's Cardiosave Hybrid and Cardiosave Rescue IABPs. Both devices were subject to a separate recall that the FDA determined was Class I in … [Read more...] about Getinge’s Datascope has another serious intra-aortic balloon pump recall
Surmodics is laying off roughly 60 workers
Surmodics (Nasdaq: SRDX) announced a restructuring that includes cutting 13% of the workforce at the Eden Prairie, Minnesota-based IVD tech developer. In its most recent annual report, Surmodics listed a headcount of 447, which means the layoff could involve nearly 60 workers. The cost-reduction plan will save roughly $10 million to $11 million … [Read more...] about Surmodics is laying off roughly 60 workers
Surmodics announces regulatory delay for its SurVeil balloon
The FDA said it needed more information on biocompatibility and labeling.
The FDA has indicated that Surmodics' Surveil drug-coated balloon premarket approval application is not approvable.
According to an FDA letter to the company, Surmodics' Surveil was not approved due to certain information in the biocompatibility and labeling categories. The governing agency said more information needs to be added by an amendment … [Read more...] about Surmodics announces regulatory delay for its SurVeil balloon
Boston Scientific to acquire majority stake in interventional tech developer Acotec
Boston Scientific (NYSE:BSX) announced that it intends to make a partial offer to acquire a majority stake in Acotec. Marlborough, Massachusetts-based Boston Scientific's offer could add up to a maximum of 65% of Acotec's shares. Its proposed price totals HK$20 per share. That amounts to approximately $523 million for the 65% stake at current … [Read more...] about Boston Scientific to acquire majority stake in interventional tech developer Acotec
The top 10 catheter innovation news stories of 2022
This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022
Surmodics beats The Street in Q4 results, grows sales 8%
Surmodics (Nasdaq:SRDX) today posted fourth-quarter results that beat the overall consensus on Wall Street. The Eden Prairie, Minnesota-based IVD tech developer reported net losses of $14.7 million, or -$1.06 per share, on sales of $25.99 million for the three months ended Sept. 30, representing a sales growth of 8.41% compared to Q4 2021. The … [Read more...] about Surmodics beats The Street in Q4 results, grows sales 8%
MedAlliance’s Selution SLR wins FDA IDE approval for in-stent restenosis
MedAlliance today announced it received FDA investigational device exemption (IDE) approval for its Selution SLR to initiate its coronary pivotal clinical trial for in-stent restenosis. Geneva-based MedAlliance's Selution SLR is a drug-eluting balloon that is indicated for below-the-knee and superficial femoral artery (SFA) indications, for … [Read more...] about MedAlliance’s Selution SLR wins FDA IDE approval for in-stent restenosis