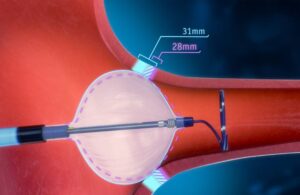
The new system received an indication for the treatment of patients with paroxysmal AFib. It features the POLARx FIT cryoablation balloon catheter. This device enables two balloon sizes (28 mm and 31 mm) all in one catheter.
Cryoablation, a minimally invasive AFib treatment, features a balloon catheter that delivers cryotherapy to the pulmonary vein. It freezes problematic tissue and creates scarring that blocks irregular electrical signals.
Marlborough, Massachusetts-based Boston Scientific designed its POLARx system to address known limitations of existing cryoablation options. Physicians can adjust and expand the new catheter to fit an individual patient’s anatomy during an ablation procedure. Boston Scientific says this can help to mitigate time-consuming and disruptive device changeouts.
The device also enables physicians to treat a wider range of pulmonary vein anatomies and create lesions in optimal positions. This allows the physician to better deliver therapy to areas of the heart in which the signals that cause AFib originate.

