 Cordis recently issued a warning letter regarding an issue with its Smart Flex vascular stent system.
Cordis recently issued a warning letter regarding an issue with its Smart Flex vascular stent system.
Santa Clara, California-based Cordis’s field safety correction action informs users that some of its Smart Flex vascular stents have the potential for distal tip dislodgment or separation due to inadequate adhesive application.
The recall warns that a distal tip separation could result in an intra-procedural delay as the device is exchanged for another, unplanned percutaneous or surgical intervention and peripheral ischemia or necrosis.
Smart Flex stents with 5 mm through 8 mm diameters treat atherosclerotic superficial femoral artery lesions and proximal popliteal lesions. The 9 mm and 10 mm diameters are intended for use in the common and external iliac arteries to improve luminal diameters in patients with symptomatic vascular stenotic or occlusive diseases.
The recall affects several lot numbers, including:
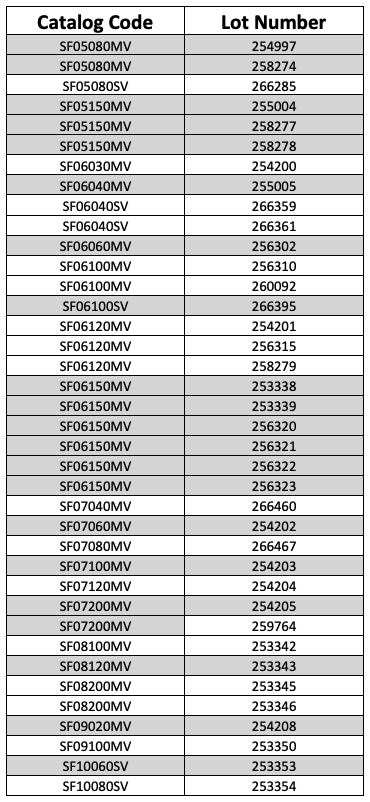
Cordis recommends that users check inventories for affected units and return all devices to the Cordis distribution center.

