Tel Aviv, Israel-based Endoron is developing an innovative solution for the endovascular repair of abdominal aortic aneurysms (AAA). The grant will support the scale-up and clinical evaluation of the Aortoseal implant that is required to bring this unique technology to the 4 million patients that are diagnosed with AAA who could also benefit from a minimally invasive treatment approach.
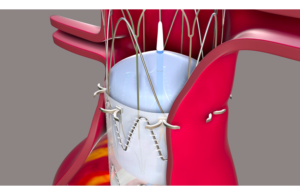
Endoron Medical developed Aortoseal to overcome the challenges associated with the sealing and fixation of endograft used for the minimally invasive repair of abdominal aortic aneurysms in difficult aortal anatomies. Aortoseal provides long-term durability to endovascular aneurysm repairs (EVAR) using its unique stapling mechanism.
“We are delighted that Endoron has been singled out by the European Commission after an extremely selective process. The support of the EIC program validates our technology and our approach, recognizing the strong value created and milestones achieved in the past 30 months. This grant will help accelerate the development and clinical validation of our Aortoseal implant, enabling a maximum of patients suffering from AAA to benefit from this minimally invasive approach, which has the potential to become the new standard of care,” CEO and cofounder Ronit Harpaz said in a news release.
Endoron Medical is one of 75 companies that were awarded funding during the latest round of the program, which had nearly 1,000 candidates.

