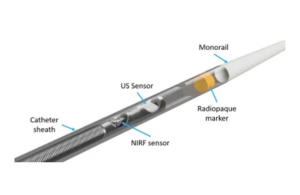
The catheter is designed in collaboration with the Massachusetts General Hospital an was tested ex-vivo in human coronary arteries and in vivo in preclinical studies. It uses intravascular ultrasound (IVUS) with near-infrared fluorescence imaging (NIRF) in a single catheter.
Wilton, Connecticut-based Intravascular Imaging Incorporated (i3) designed the catheter to enable visualization of the molecular and cellular details that underline pathophysiologic progression of coronary lesions, arterial dissections, coronary transplant vasculopathy and fibrin production in unhealed stents.
“Through the use of molecular and pathophysiologic imaging we can now visualize plaque inflammation in living subjects simultaneously with IVUS-detected plaque anatomy. Plaque inflammation is a critical driver of coronary and stent events, particularly among patients with high residual plaque inflammation on maximal statin therapy,” said Dr. Farouc Jaffer, an interventional cardiologist at Massachusetts General who founded i3. “Thus, it’s important to explore new molecular imaging approaches to personalize therapies aimed at preventing a serious, life-threatening cardiac events.”
The company was previously funded through federal and foundational grants and industry sponsorship from a leading imaging manufacturer and is now raising its first equity round.
“With these extraordinary early preclinical and ex vivo human results, i3 will raise its first equity round, advance its GMP manufacturing and position its device for a pivotal human study and eventual FDA submission,” CEO Scott Jones said in a news release. “We will market our product as an upgrade to the fast-growing international IVUS marketplace.”

