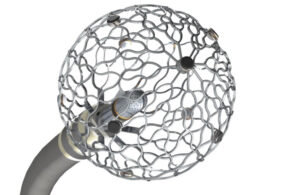
The trial is evaluating the safety and effectiveness of the Sphere-9. The Sphere-9 is a pulsed field (PF) and radio frequency (RF) ablation and high-density mapping catheter. Along with the Affera cardiac mapping and navigation platform, it treats atrial fibrillation.
Affera designed the technology to diagnose, map and treat heart arrhythmias with ablation. It scars heart tissue to interrupt errant signals using both radiofrequency ablation and non-thermal pulsed-field ablation technology. Medtronic acquired the company for $1 billion in August.
More about Medtronic Sphere Per-AF pivotal trial
The Sphere-9 catheter uses mapping, navigation and therapeutic capability to deliver RF and PF energies in one catheter for ablation purposes. It gives physicians the ability to customize treatment based on a patient’s needs during an ablation procedure.
“During the trial, my observations and experience with the novel Affera system have been very promising,” said Dr. Devi Air, director of cardiac electrophysiology and research at St. Bernard’s Medical Center in Jonesboro, Arkansas. “Unlike conventional technologies, I’ve been impressed with the ability to both map and ablate with the option of dual energy sources, with one catheter. I look forward to the results of the trial and remain optimistically enthusiastic as I continue to understand the safety and efficacy of the Sphere 9 ablation catheter.”
Sphere-Per-AF trial is a global, prospective, multi-center, randomized clinical trial. Medtronic has enrolled 477 patients with persistent AF across 23 centers in the U.S. and Europe. Researchers will assess patients for 12 months for safety and efficacy.
“Treating the final patient in the fast-moving Sphere Per-AF Trial builds on the exciting phase of innovation and growth at Medtronic over the last year, including the acquisition of Affera, our agreement to distribute a differentiated portfolio of left-heart access tools and devices to support a zero-exchange procedure workflow, and the continued progress in the development of PulseSelect, our organic PFA system,” said Rebecca Seidel, president of cardiac ablation solutions at Medtronic. “Thanks to the innovation and expertise within Affera and the support of our Medtronic team, together we’re able to continue to evaluate new, best-in-class solutions and commercialize a full, comprehensive portfolio to help physicians treat patients around the world.”
Affera is not currently approved or available for sale or commercial use.

