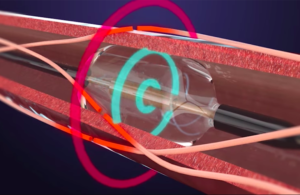
The first commercial uses follow the company’s landmark FDA approval for Paradise last week. Approval allows for the use of Paradise as an adjunctive treatment option when lifestyle changes and medications fail to control a patient’s blood pressure adequately.
Recor edged out Medtronic in the race to offer the first FDA-approved RDN system that treats hypertension.
The first procedures took place in New York, Ohio and in California. Dr. Ajay J. Kirtane and Dr. Sahil A. Parikh conducted procedures at NewYork-Presbyterian/Columbia University Irving Medical Center. At the Smidt Heart Institute at Cedars-Sinai in Los Angeles, Dr. Suhail Dohad and Dr. Raj Makkar conducted theirs. Dr. Vijay Iyer and Dr. Aravinda Nanjundappa also performed procedures at Gates Vascular Institute in Buffalo, New York, and Cleveland Clinic in Cleveland, Ohio, respectively.

