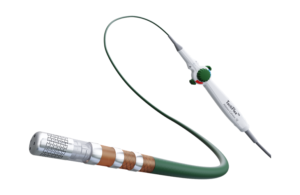
Abbott designed the catheter to treat people suffering from paroxysmal atrial fibrillation (PAF) with symptoms that are unable to be managed by medication.
TactiFlex combines Abbott’s contact force-sensing technology within the TactiCath SE catheter with a catheter tip design found in the company’s Flexibility irrigated ablation catheter. The combination of the two technologies allows for an appropriate amount of pressure to the heart walls with proper fluid rates to cool the tissues, according to Abbott.
Abbott’s TactiFlex PAF IDE study will enroll 355 patients at multiple sites globally. Participants will receive an ablation procedure using the TactiFlex Ablation Catheter SE. Abbott will submit data collected from the study for global regulatory approvals.
“The TactiFlex device builds on prior generations of successful technology, giving me confidence that Abbott will continue providing options to effectively treat people living with recurrent episodes of atrial fibrillation,” said Prash Sanders, director for the Center for Heart Rhythm Disorders at The Royal Adelaide Hospital in Adelaide, Australia.
Atrial fibrillation is the most common type of treated heart arrhythmia, according to the Centers for Disease Control and Prevention. It is estimated that between 2.7 million and 6.1 million people have AFib in the U.S.
“Abbott is focused on bringing innovation to physicians that can improve how people with atrial fibrillation are treated. The TactiFlex Ablation Catheter, SE brings the best of Abbott’s ablation technologies into a single catheter,” said Mike Peterson, senior VP of Abbott’s electrophysiology and heart failure businesses.
The TactiFlex sensor-enabled ablation catheter is currently under clinical evaluation and is not yet available commercially.
