 BD today said it has received FDA 510(k) clearance for its PeritX peritoneal catheter system.
BD today said it has received FDA 510(k) clearance for its PeritX peritoneal catheter system.
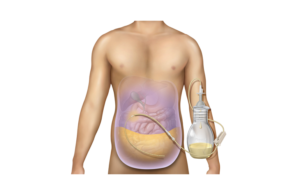
Franklin Lakes, N.J.-based BD designed the catheter system to drain symptomatic, recurrent non-malignant ascites, which causes a build-up of fluid in the abdomen.
The company’s PleurX peritoneal catheter system was first introduced in 2005 to drain malignant ascites. With the expanded FDA clearance, the company is rebranding the system to PeritX.
“The new, expanded indication for the PeritX peritoneal catheter system will allow us to help more patients who present with ascites due to liver disease or other noncancer-related origins,” Padraic O’Brien, worldwide president of peripheral intervention, said in a news release. “Recurrent ascites is a debilitating condition that requires the patient to make frequent trips to the hospital so that fluid can be removed from the peritoneum. The PeritX peritoneal catheter system allows patients to manage non-malignant ascites at home and aligns with our goal to help keep patients out of the hospital.”

