 BD has issued an urgent field safety notice for its Venovo venous stent system.
BD has issued an urgent field safety notice for its Venovo venous stent system.
The notice out of Germany warns of potential delayed deployment and silicone embolization if the proximal end of the Venovo venous stent system does not immediately expand upon deployment and remains connected to the stent cushion on the delivery system.
According to BD, there is no incremental risk of harm in cases where the stent self-expands. However, over-manipulation or forcing the catheter delivery system in attempts to assist the stent’s expansion could potentially have a varying degree of harm associated with it. Potential harm ranges from prolonging the procedure, damaging or deformity of the stent, potential vascular injury or hemodynamic disruption affecting the blood flow or a thrombotic event.
There have been no reported complaints to date across the product codes and lot number combinations.
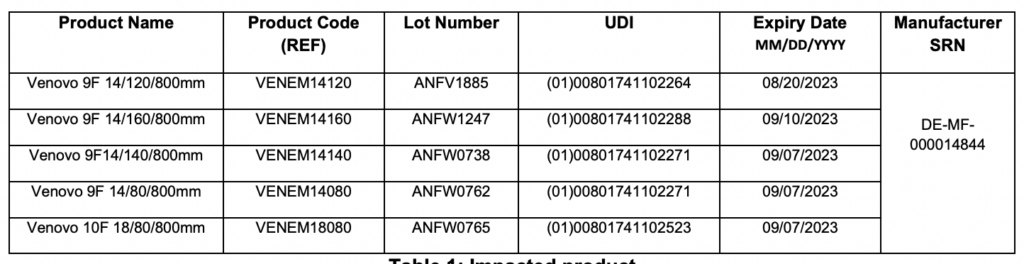
Lots affected by the urgent field safety notice include:
BD said the hazardous situation associated with the Venovo venous stent is that it, in a focal area near its proximal end, does not immediately expand at the time of deployment. As a result, the physician may not allow enough time for normal expansion and could try to manipulate the stent or use other intravascular devices or techniques to help expand the stent.
These risks could lead to misplacement or damage to the stent and vascular injury. A transfer of medical-grade biocompatible silicone adhered to the inner surface of the stent could lead to inflammatory responses or blockage/obstruction of the vasculature.
BD advises customers to cease use of any unused affected Venovo venous stent systems and quarantine all unused, affected devices. A BD representative will contact the customer on the next steps.

