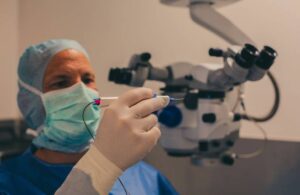
The FDA cleared iTrack Advance for microcatheterization and viscodilation to reduce intraocular pressure (IOP). It treats adult patients with primary open-angle glaucoma.
Fremont, California-based Nova Eye designed its newest canaloplasty device to leverage the proprietary features of its original iTrack. That includes a 200-micron illuminated canaloplasty microcatheter. However, the new design aims for improved surgical efficiency with an ergonomic handpiece. Additional improvements to the platform include proprietary illuminated microcatheter technology.

