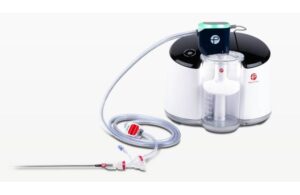
Lightning Flash, a mechanical thrombectomy system, received FDA clearance in January. The system features Penumbra’s novel Lightning intelligent aspiration technology with dual-clot detection algorithms. Combining it with innovative catheter engineering, Penumbra designed Lightning Flash to quickly remove large blood clots, including venous thrombus and pulmonary emboli (PE).
This combination can differentiate between clot and blood. Its design, which helps reduce blood loss and the need for clot-dissolving drugs, may lower the risk of bleeding complications.
The trial evaluates anticoagulation alone against anticoagulation plus Lightning Flash for the treatment of PE. STORM-PE, a first-of-its-kind, multi-center, randomized controlled trial, aims to advance the understanding of the role of computer-assisted vacuum thrombectomy (CAVT) in the management of acute PE. Penumbra said it hopes to improve the outcomes of patients with the life-threatening condition.
According to a news release, Penumbra plans to enroll up to 100 participants at up to 20 sites. Dr. Rachel Rosovsky of Massachusetts General Hospital, the co-global principal investigator, said it marks the first head-to-head trial comparing these methods. Rosovsky said Lightning Flash has already proven to have an impact but results will see how CAVT compares to current paradigms.
“Our commitment to clinical research and innovation enables us to lead with insight and continue to pioneer interventional therapies that have a significant impact on patients such as our CAVT technologies,” said Dr. James F. Benenati, chief medical officer at Penumbra. “In partnership with PERT Consortium, we are committed to generating robust clinical evidence that will help transform care so patients, especially those with serious conditions such as PE, can return home quickly and live fully.”

