Hercules compares endosuture aneurysm repair (ESAR) to standard endovascular aneurysm repair (EVAR). It looks at these methods in patients who have an abdominal aortic aneurysm (AAA) with a wide, infrarenal neck diameter.
Abdominal aortic aneurysms occur in about 1.4% of people ages 50 to 84 in the U.S., according to Cleveland Clinic. Aging, tobacco use and certain medical conditions can cause the aorta’s wall to weaken and bulge. A burst is life-threatening. Over more than a decade, Medtronic’s Endurant stents have grown as an alternative to open surgery.
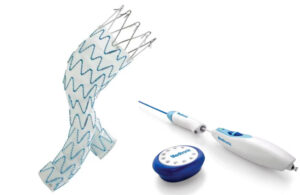
Patients in the Hercules trial will receive either the Endurant II/IIs stent graft (EVAR arm) or the Endurant II/IIs stent graft in conjunction with the Heli-FX EndoAnchor system (ESAR arm). The Heli-FX EndoAnchor provides transmural fixation of the endograft to the aorta in order to help reduce later complications.
Medtronic plans to recruit a total of 300 patients worldwide. It expects the trial to take place at approximately 20 hospitals in Europe and 20 in the U.S.
According to a news release from Rijnstate Hospital, both the condition and surgery carry risks. Many aneurysms require minimally invasive treatment featuring a stent graft inserted into the abdominal artery. The area where the stent graft attaches under the renal artery is referred to as the “neck.”
Dr. Michel Riejnen, the principal investigator for the Hercules trial, says about a quarter of all patients with an aneurysm have a wide neck. This means the top of the aneurysm registers wider than normal. That can increase the risk of longer-term complications after surgery through the groin.
Risks can include the displacement of the stent and leakage in the aneurysm. That increases the risk of rupture again, often leading to another surgery.
“By securing the stent graft to the vessel wall in the neck with specially developed implants, called EndoAnchors, we try to prevent complications and associated re-operations. Next, we must investigate whether this is really the case,” the doctor said. “We will do that in the coming years.”

