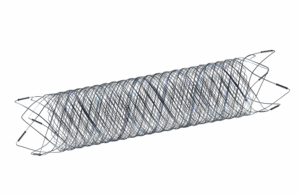
Terumo Neuro’s MicroVention issued an urgent medical device recall of some Fred 27 and Fred X 27 Flow re-direction endoluminal devices.
The company is voluntarily recalling certain lots of the devices due to complaints of a manufacturing error related to the tantalum length and attachment pattern. Terumo Neuro stated that the complaints have also reported secondary interventions to prevent patient injury.
Incorrect tantalum wire length or attachment can lead to delivery or deployment problems during implantation, the notice said. If a device does not deploy correctly, physicians may need to recapture the device and withdraw the delivery system and microcatheter, which could delay the procedure. Deployment problems may also result in stents being implanted with insufficient apposition if not detected, which could have long-term health consequences.
Terumo Neuro said that if the device was implanted and there was no indication that it was incompletely open or not properly apposed, there is no additional risk beyond the baseline risk of a neurovascular flow diversion procedure.
In a statement emailed to Medical Tubing + Extrusion, Terumo Neuro said: “At Terumo Neuro, patient safety is always our top priority. We recently issued a field safety notice regarding a very limited number of FRED 27 devices after identifying a potential issue related to tantalum wire length and attachment. While the number of impacted units is extremely small, we acted quickly and transparently out of an abundance of caution to ensure physicians had the necessary information. We continue to work closely with regulatory authorities and physician partners to ensure patient safety and our mission of improving patient outcomes globally.”
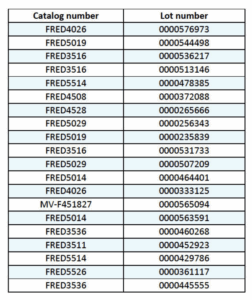
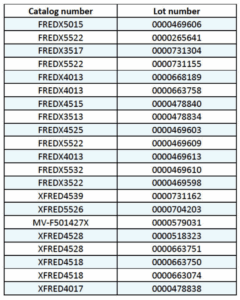
Specific recalled lots are listed below: