That’s the most serious level of a medical device recall, carrying the risk of serious injury or death. Five incidents, one injury and zero deaths were reported when Abbott initiated the recall on April 11.
The recall affects 4,800 devices in the U.S. from certain lots. Abbott is asking health care providers to stop using the devices.
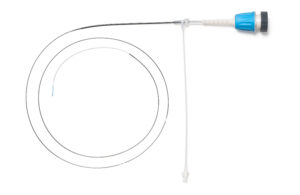
Abbott’s Dragonfly OpStar Imaging Catheter and its optical coherence tomography (OCT) imaging system are designed to provide imaging of blood vessels that carry blood and oxygen to the heart in patients who are candidates for minimally invasive catheter-based procedures for coronary artery disease.
Abbott warned health care providers that the catheter’s proximal marker band (the one farthest from the catheter tip) may become loose and separate while being used on a patient. This has been observed at least twice, the FDA said.
The FDA said yesterday that a loose marker band that separates from the device can remain in the body after the catheter is removed, which could lead to vascular injuries such as embolism, thrombosis, dissection, ischemia, infarction, infection or death.

