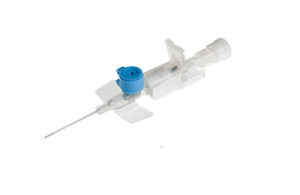
BD (NYSE: BDX) has issued a recall for certain lots of its Venflon Pro IV cannula due to the potential for leakage from the injection port.
The company declared the latest recall “out of an abundance of caution” following a different voluntary recall for specified lots of BD Venflon Pro Safety Needle Protected IV cannula that shares the same valve design.
The recall comes after the company decided to switch from e-Beam to ethylene oxide (EtO) sterilization for the cannulae. In order to make the switch, BD had to change the injection port valve dimension, company spokesperson Troy Kirkpatrick said in an email to Medical Tubing + Extrusion.
“BD changed the sterilization method from E-Beam to EtO in November 2019 to bring product sterilization in-house, capitalizing upon excess internal EtO sterilization capacity at the time in one of our European facilities,” he explained. “BD is currently taking steps to investigate and implement corrective actions.”
The recall covers 14 different catalog numbers for BD Venflon Pro IV cannula peripheral IV catheters distributed in eastern and western Europe, Asia, the Middle East and Russia, Kirkpatrick said. This recall does not affect the U.S.
Kirkpatrick said adverse events have been reported to the appropriate national competent authorities, but he did not reveal how many. No deaths have been reported and no additional follow-up activities are required for patients already treated with the devices, Kirkpatrick added.

