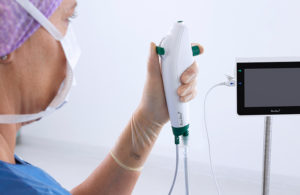
Light Line Medical today said it entered into a "know-how" license agreement with Mayo Clinic to advance its visible light infection prevention system for off-the-shelf catheters. The collaboration will focus on the technology in peritoneal dialysis catheters, Foley (urinary) catheters and endotracheal tubes and vascular catheters. It will also … [Read more...] about Light Line Medical enters licensing agreement with Mayo Clinic for catheter sterilization system
Sterilization
Study finds single-use bronchoscopes reduce hospital readmission rates by half
A study of 14,228 bronchoscopy procedures found sterile, disposable scopes reduced readmission rates by more than half. The study, authored by Ambu consultant Dr. Hudson Garrett, analyzed procedures at inpatient hospitals as well as outpatient ambulatory facilities. He found that 3.6 percent of patients treated with a single-use, flexible … [Read more...] about Study finds single-use bronchoscopes reduce hospital readmission rates by half
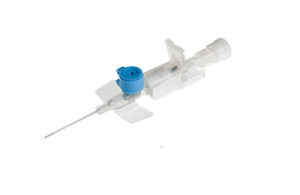
BD recalls certain IV cannulae in Europe, Asia
BD (NYSE: BDX) has issued a recall for certain lots of its Venflon Pro IV cannula due to the potential for leakage from the injection port. The company declared the latest recall "out of an abundance of caution" following a different voluntary recall for specified lots of BD Venflon Pro Safety Needle Protected IV cannula that shares the same … [Read more...] about BD recalls certain IV cannulae in Europe, Asia
5 innovations to make duodenoscopes more single-use — and save lives
Duodenoscopes are important medical devices that are used for endoscopic retrograde cholangiopancreatography (ERCP) procedures. However, the devices have had serious problems in the past. Luckily, several medical device companies have stepped in to solve the problems. (Here are five of the innovations.) Duodenoscopes are flexible, lighted … [Read more...] about 5 innovations to make duodenoscopes more single-use — and save lives
How glass tubing supports Mayo Clinic innovation
At the Mayo Clinic Glass Shop, scientific glassblowing creates glass tubing and apparatuses for cardiac, transplantation and tissue perfusion research — and more recently, to help fight the COVID-19 pandemic. Steve Anderson is one of only two scientific glassblowers in Minnesota. As a senior scientific glassblower at Mayo Clinic, he has … [Read more...] about How glass tubing supports Mayo Clinic innovation
Teleflex warns on Arrow multi-lumen catheter
Teleflex (NYSE:TFX) this week initiated a voluntary field safety corrective action in Europe for its Arrow subsidiary's multiple-lumen catheter. In a field safety notice dated March 16, Teleflex announced that Arrow is voluntarily recalling the catheter due to a potential sterility issue. The catheter's product tray may be damaged in affected … [Read more...] about Teleflex warns on Arrow multi-lumen catheter
FDA issues draft guidance on multi-use arthroscopy tubing sets
The FDA has issued draft guidance on arthroscopy pump tubing sets intended for multiple-patient use. Clinicians often use a single source of irrigation fluid for multiple patients without replacing the source of irrigation fluid or replacing/reprocessing the irrigation tubing system between patients. These devices are designed to deliver … [Read more...] about FDA issues draft guidance on multi-use arthroscopy tubing sets
EO plant shutdown leads to pediatric breathing tube shortage
The February shutdown of an ethylene oxide (EO) sterilization plant has produced the first temporary medical device shortage, according to the FDA. The device in short supply is the Bivona tracheostomy tube manufactured by Smiths Medical and used by many pediatric patients. The FDA anticipates the tube will be made available again the week of … [Read more...] about EO plant shutdown leads to pediatric breathing tube shortage
How to streamline sterile package validation
The requirements for validating the back-end processes for packaging, manufacturing, sterilization and shelf life of a sterile medical device are often misunderstood. Errors and oversights can result in failed validation tests and longer-term sterile barrier reliability. Jeff Barrett, J-Pac Medical It can take nearly a year to get a validated … [Read more...] about How to streamline sterile package validation
New study demonstrates 87% of rigid containers tested allowed bacterial contamination, and more
According to a new scientific study published in the December print publication of the American Journal of Infection Control (AJIC),i 87 percent of tested sterilized rigid containers – used in the sterilization of surgical instruments – allowed ingress of aerosolized bacteria under the test conditions used. This study calls into question the … [Read more...] about New study demonstrates 87% of rigid containers tested allowed bacterial contamination, and more