Our previous roundup of the top 10 most-read catheter-based news stories of 2023 wasn’t enough for us. Medical Tubing + Extrusion‘s editors could not stop thinking about several other stories when considering the medical device innovations of the year.
It was a year of many firsts for some companies. For example, Recor Medical was the first company to win FDA approval for renal denervation, and Medtronic was the first to win FDA approval for pulsed-field ablation – both treatment options that we’ve set our sights on for the last few years. While readers were interested in cardiology-based catheter-based innovations, we thought many more critical applications should not be missed.
The editorial team at Medical Tubing + Extrusion drew on its resources and knowledge of the news coverage in the industry to create this roundup of catheter-based stories that we thought were most impactful in medtech this year. From technology that treats chronic limb-threatening ischemia to the first pulsed-field ablation approval in the U.S., here are some of the top news stories our editors can’t stop thinking about, in no particular order.
Recor Medical wins first FDA nod for renal denervation to treat hypertension
November 8, 2023 | By Sean Whooley
Recor Medical announced that the FDA approved its Paradise ultrasound renal denervation (RDN) system for treating hypertension.
The long-awaited approval for the Otsuka Medical Devices portfolio company allows for the use of Paradise as an adjunctive treatment option when lifestyle changes and medications fail to adequately control a patient’s blood pressure.
Paradise had looked the most likely system to win approval in the race for the first FDA nod to use RDN to treat hypertension. An FDA panel voted in favor of FDA approving Paradise uRDN system in August. The same panel voted against recommending Recor’s closest competition, Medtronic’s Symplicity Spyral, a day later.
Read more about Recor’s FDA nodWhat our editors are saying

FDA approves Medtronic’s Symplicity Spyral renal denervation system
November 17, 2023 | By Jim Hammerand
Medtronic announced FDA approval of its Symplicity Spyral renal denervation (RDN) system for treating hypertension.
A Medtronic spokesperson said the device developer received word from regulators Friday evening. Medtronic is now the second company with premarket approval (PMA) to market an RDN system for hypertension in the U.S., following Recor Medical’s approval earlier this month.
Medtronic said it plans to immediately commercialize its system and the Symplicity blood pressure procedure.
Read more about the FDA approvalWhat our editors are saying
Medtronic PulseSelect pulsed field ablation wins FDA approval
December 13, 2023 | By Jim Hammerand
The FDA has approved the Medtronic PulseSelect pulsed field ablation (PFA) system, the device developer said today.
PulseSelect is the first PFA technology approved for use in the U.S., as well as the first PFA technology with FDA breakthrough designation to win approval.
The minimally invasive, cardiac ablation system is indicated for the treatment of paroxysmal and persistent atrial fibrillation (AFIb).
Medtronic said it will start commercialization in early 2024.
Read more about the approvalWhat our editors are saying
Endolumik’s illuminated device takes a big step for safety
March 21, 2023 | By Jim Hammerand
Endolumik’s story started a few years ago as the FDA sounded the alarm over the risks of internal surgical staplers.
In 2019, the federal agency warned healthcare providers that it had received more than 41,000 medical device reports (MDRs) related to surgical staplers and staples for internal use from 2011 to 2018. Those MDRs tallied more than 32,000 malfunctions, at least 9,000 serious injuries and 366 deaths.
Two years later, the FDA increased the risk level associated with the staplers, reclassifying the devices from Class I to Class II and subjecting them to premarket review and special controls.
Bariatric surgeon Dr. Nova Szoka had already been working on a device that could prevent some of those injuries. She envisioned an illuminated gastric calibration tube that would shine near-infrared light through stomach tissue and fat. This camera-visible fluorescence would make it easier to see the tube inside a patient, lessening the risk of a surgeon perforating the stomach or stapling the tube to the stomach during bariatric procedures.
Read more on MDOWhat our editors are saying
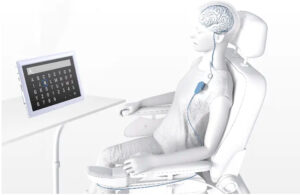
Opening the brain’s secret back door: A conversation with Synchron co-founder and CEO Dr. Tom Oxley
December 11, 2023 | By Jim Hammerand
What seems like a miracle today — a paralyzed patient regaining the ability to communicate with their family without open-brain surgery — may eventually seem obvious in retrospect.
It already does to Dr. Tom Oxley, the interventional neurologist who’s CEO and co-founder of brain-computer interface (BCI) developer Synchron.
Synchron’s Stentrode device is implanted inside a blood vessel in the brain to sense neural signals and relay them to another implant in the chest. Those signals are then translated into digital commands, allowing the patient to communicate electronically or control smart devices.
The FDA has given breakthrough device designation to the system, which Synchron is testing in six patients through its COMMAND trial. It’s the first FDA investigational device exemption (IDE) for any company assessing a permanently implanted BCI.
Read more about the BCI technologyWhat our editors are saying

How LimFlow’s foot-saving system prevents amputations in patients with no other options
September 22, 2023 | By Jim Hammerand
LimFlow’s breakthrough system for treating chronic limb-threatening ischemia (CLTI) is the first of its kind approved by the FDA for this severe form of peripheral artery disease (PAD).
For CLTI patients who have lost blood flow below their knee and have no other suitable endovascular or surgical treatment options available, the LimFlow System for Transcatheter Arterialization of Deep Veins (TADV) is now the last resort to avoid amputation.
LimFlow’s TADV system won breakthrough device designation in October 2017 and secured FDA premarket approval (PMA) in September 2023.
Read more hereWhat our editors are saying

